Abstract
The genus Diplazium (family: Athyriaceae) comprises approximately 350 species of pteridophytes. Diplazium esculentum (Retz.) Sw. is an important member of this genus and commonly known as a wild vegetable in the Himalayan and sub-Himalayan communities. According to the literature analysis, D. esculentum was traditionally used for the prevention or treatment of several diseases such as diabetes, smallpox, asthma, diarrhea, rheumatism, dysentery, headache, fever, wounds, pain, measles, hypertension, constipation, oligospermia, bone fracture, and glandular swellings. Various extracts of D. esculentum were evaluated to elucidate their phytochemical and pharmacological activities. A wide array of pharmacological properties such as antioxidant, antimicrobial, antidiabetic, immunomodulatory, CNS stimulant, and antianaphylactic activities have been recognized in different parts of D. esculentum. The review covers a systematic examination of pharmacognosy, phytochemistry, and pharmacological applications of D. esculentum, but scientifically, it is not fully assessed regarding complete therapeutic effects, toxicity, and safety in the human body. The published literature on D. esculentum and its therapeutic properties were collected from different search engines including Wiley online, PubMed, Springer Link, Scopus, Science Direct, Web of Science, Google Scholar, and ACS publications by using specific terms such as “Diplazium esculentum, bioactive compounds, biological activities and health benefits” from 1984 to 2021 (March). Therefore, further studies are required to identify the detailed action mechanism of D. esculentum in vitro/in vivo, and also, more studies should focus on conservation, cultivation, and sustainable utilization of the species.
1. Introduction
The Himalayan botanicals are well known to produce wide variety of secondary metabolites due to critical climatic conditions [1–4]. These botanicals, including wild plants, have a significant role in food security and socio-economic development of the region [5, 6]. Moreover, these botanicals are locally utilized for food resources, medicines, and other purposes due to the presence of numerous bioactive compounds and high nutritional value [7, 8]. With recent developments in science and technology, the importance of wild plants has been identified as a possible source of nutraceuticals and/or functional foods [9].
Among several high valued functional foods, Diplazium esculentum is one of the important species of wild ferns, which is frequently consumed by people living in the hilly areas; it is not growing on much higher altitude. D. esculentum (n = 41 chromosomes, grade of polyploidy = diploid) is utilized as a traditional vegetable in the Himalayan communities [10]. It is an important member of the genus Diplazium which comprises around ~350 species of pteridophytes, mainly distributed in Asia and Oceania [11].
Specifically, D. esculentum is distributed through different parts of the globe including Cambodia, China, India, Indonesia, Japan, Malaysia, Papua New Guinea, Pakistan, Philippines, Singapore, Taiwan, Thailand, Vietnam, and Bangladesh. It grows on the banks of rivers and streams, canals, marshy areas, and hills with an altitudinal range up to 2,300 meters [12, 13].
It is locally known by different names such as English: vegetable fern; India: dung-kek, kari-welli-panna-maravara, kasrot, kukari-sag, mairungshai, para-panna-maravara, linguda, kathura; Japan: Kuware-shida; Malay: paku, paku-tanjong; Nepali: paninyuro, piraunli; Papua New Guines: sigogo; Philippines: Pako; Thai: kut-kin; and Bangladesh: Dheki Shak [14].
Traditionally, D. esculentum is being used in the treatment of various ailments (as shown in Figure 1) such as diabetes, smallpox, asthma, diarrhea, rheumatism, dysentery, headache, fever, wounds, pain, measles, high blood pressure, constipation, oligospermia, bone fracture, glandular swellings, and skin-related diseases by the different communities in India and other countries [15–21].
Figure 1.
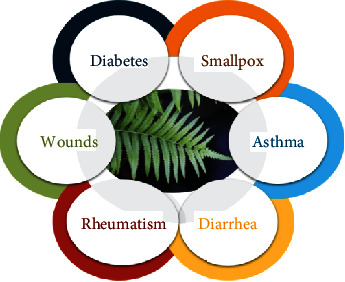
Traditional uses of Diplazium esculentum.
Recently, a few researchers have validated its nutraceutical and pharmacological properties by using in vitro and in vivo models/methods. For the development of evidence-based medicine, a critical investigation of current knowledge is required regarding ethnopharmacology, chemical composition, biological activities, and possible side effects of the species. Additionally, D. esculentum belongs to the least concern category under International Union for Conservation of Nature (IUCN) 2021-1 (https://www.iucnredlist.org/species/194150/8883499) and needs more attention. Therefore, in this manuscript, we reviewed and discuss the recent scientific information conducted so far on D. esculentum, which includes its pharmacognosy, phytochemistry, and pharmacology.
2. Pharmacognosy
2.1. Traditional Uses
Traditionally, D. esculentum is one of the most popular vegetables consumed in different parts of the globe, namely, India, Philippines, Nepal, China, Thailand, Indonesia, etc. The literature has revealed that D. esculentum is still being used by different communities for the treatment of several diseases including diabetes, smallpox, asthma, diarrhea, rheumatism, dysentery, headache, fever, wounds, pain, measles, and high blood pressure. The detailed information on the traditional uses of the species is summarized in Table 1. Additionally, this species is collected in large amounts and marketed by the rural and tribal communities of India for their livelihood enhancement [22]. The Mishing community of Assam (State of the Indian republic) used it essentially in the religious ceremony of the dead person [23].
Table 1.
Traditional uses of D. esculentum in different regions.
| Plant part | Ethno-pharmacological uses | Country | References |
|---|---|---|---|
| Fronds/leaves/areal part/whole plant | Cooked and eaten as a vegetable and in soups to maintain good health. | India, Bangladesh, Thailand | [23, 36–38] |
| Tender leaves are cooked with fruit of Dillenia indica and fish and taken as vegetable. | Malaysia, India | [39, 40] | |
| Hairs are removed, boiled with salt and water until water is evaporated then fried and eaten as vegetable. | India | [41–45] | |
| Used in headache, pain, fever, wounds, dysentery, glandular swellings, diarrhea, measles, toothache, high blood pressure, and various skin infections. Fronds used by pregnant women as protection against difficult childbirth. Leaf paste is used in the wounded place externally for the cure of bone fracture. Used as a laxative. Used as insecticides. | Bangladesh, Nigeria, Indonesia, Nigeria, India, Philippines | [15–17, 19, 46–48] | |
| The tender frond is cooked without salt and is consumed with rice for 5–10 days for the treatment of diabetes. | India | [20] | |
| Eaten as highly preferred Koche Sag, Neuro/Niuro vegetable. | Nepal | [49] | |
| Used as vegetable and pickle. | India, Vietnam, Japan, Indonesia, Philippines | [30, 31, 50–53] | |
|
| |||
| Root | About 20 g of fresh root is boiled in 1 liter of water and reduced to one-fourth of its volume. 3 mL of this decoction along with 2 mL of honey is taken orally on an empty stomach twice a day for 15 days to cure spermatorrhea. | India | [54] |
| About 50 g juice obtained from macerated root is fed three times for human dysentery. Macerated root extract is also useful for the cattle dysentery. | Bangladesh | [55] | |
| About 2-3 spoonsful of root juice are taken for 1/2 days, or 1/½ cup of boiling extract of whole plant is taken thrice daily to treat infections and used as an antidote. The root paste is used externally for the treatment of rheumatism and smallpox. Two pills of pulverized root and honey are taken thrice daily for 2 weeks for the treatment of oligospermia. | Bangladesh | [17] | |
|
| |||
| Rhizome | Decoction of rhizome used as a tonic and also used for the cure of hemoptysis and cough. | India | [39, 56] |
2.2. Proximate and Mineral Composition
The nutritional value of any food substance can be analyzed by its proximate and mineral composition [24]. Literature-based screening of the proximate composition of D. esculentum revealed the presence of lipids, proteins, carbohydrates, vitamins, fiber, etc., while mineral composition possesses the presence of essential micro and macro compounds [25–35]. The comparative analyses of proximate and mineral composition of D. esculentum are presented in Tables 2 and 3.
Table 2.
Proximate composition of Diplazium esculentum from different regions.
| Parameters | Bangladesh (mg/100 g) [34] | Indonesia (%) [29] | India (%) [26] | India (%) [57] | Philippines (%) [33] | Nepal (%) [58] | Indonesia (%) [27] | India (%) [59] | India (%) [60] |
|---|---|---|---|---|---|---|---|---|---|
| Moisture (%) | 8.8 | — | 89.34 | 92.4 | 91.82 | 93.25 | 90.84 | 93.1 | 90.4 |
| Lipid | 2.16 | — | — | — | — | — | — | — | — |
| Protein | 8.73 | 6.20-8.30 | 3.84 | 31.2 | 0.87-10.67 | 0.99 | 2.23 | 2.6 | 8.87 |
| Ash | 5.09 | 1.90-2.11 | 1.33 | 16.2 | 1.42-17.39 | 1.10 | 1.38 | 1.3 | — |
| Total carbohydrate | 59.62 | — | — | 44.3 | — | — | — | 1.0 | 18.8 |
| Fiber | 15.59 | — | 5.05 | 4.6 | 0.72-9.06 | 0.99 | 4.82 | — | 3.1 |
| Fat | — | 0.51-0.68 | 0.25 | 8.3 | 0.28-3.40 | 0.15 | 0.04 | 2.0 | 2.5 |
| Water level | — | 2.70-3.08 | — | — | — | — | — | — | — |
| Vitamin C (mg/100 g) | — | — | 21.38 | 21 | — | 6.20 | — | — | — |
Table 3.
Mineral composition of Diplazium esculentum from different regions.
| Parameters | Bangladesh (mg/100 g) [32] | Bangladesh (mg/g) [34] | India (mg/100 g) [26] | Indonesia (μg/g) [31] | Malaysia (mg/kg) [28] | India (mg/100 g) [57] | India (mg/g) [25] | Nepal (mg/100 g) [58] | Indonesia (mg/kg) [27] | India (mg/100 g) [59] |
|---|---|---|---|---|---|---|---|---|---|---|
| N | — | 13.97 | — | — | — | — | — | — | — | |
| P | 48 | 1.58 | — | — | — | — | 117 | 0.09 | — | |
| K | — | 7.93 | — | — | — | 914.4 | — | 0.24 | 927.4 | |
| Ca | 9 | — | 0.66 | — | — | 192.7 | — | 0.39 | 200.5 | |
| Mg | 11 | — | 9.56 | — | — | 0.36 | 10-12.11 | — | 0.14 | — |
| Fe | — | — | 14.38 | 15.7 | — | 11.2 | 20.2-23.4 | 1.03 | 44.6 | — |
| Mn | — | — | 11.91 | 7.03 | 3.24-22.5 | — | 0.04-0.38 | — | — | — |
| Na | 54 | 20.21 | 0.50 | — | — | 9.5 | — | — | 8.1 | |
| Cu | — | — | 13.37 | 3.99 | 3.24-24.3 | 0.32 | 1.03-1.28 | — | 4.24 | — |
| Al | — | — | 58.5 | 18.3 | — | — | 0.10-0.73 | — | — | — |
| As | — | — | 14.6 | — | — | — | — | — | — | — |
| Cd | — | — | 0.4 | — | — | — | — | — | — | — |
| Hg | — | — | 0.07 | — | — | — | — | — | — | — |
| Li | — | — | 2.1 | — | — | — | — | — | — | — |
| Ni | — | — | 24.5 | — | — | — | — | — | — | — |
| Pb | — | — | 0.8 | 2.46 | 0.31-3.26 | — | — | — | — | — |
| Cr | — | — | — | 0.05 | 1.19-3.03 | — | — | — | — | — |
3. Bioactive Compounds
Traditionally, botanicals are being widely used to cure various ailments due to the presence of high-valued bioactive compounds [61, 62]. Literature-based screening for bioactive compounds of D. esculentum revealed the presence of alkaloids, flavonoids, glycosides, phenolic, tannins, terpenoids, steroids, carbohydrates, fats, and oils in different solvent systems [2, 35, 46, 50, 60, 63–69].
In the study of Essien and coworkers [16], the chemical composition of essential oil isolated from D. esculentum leaves and the major volatile compounds were identified as β-pinene (17.2%), α-pinene (10.5%), caryophyllene oxide (7.5%), sabinene (6.1%), and 1,8-cineole (5.8%) (Figure 2). The essential oil of this species was composed of monoterpene hydrocarbons, oxygenated sesquiterpenoids, sesquiterpene hydrocarbons, oxygenated monoterpenoids, and nonterpene derivatives.
Figure 2.
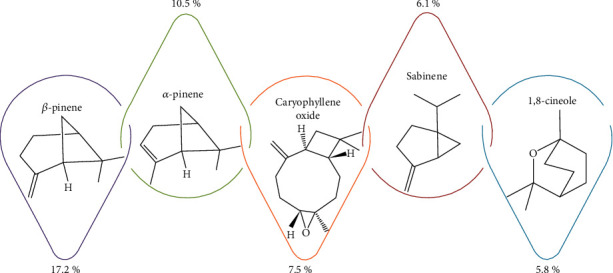
Main essential oil components of Diplazium esculentum [16].
Few compounds such as ascorbic acid [70], eriodictyol 5-O-methyl ether 7-O-β-D-xylosyigalactoside [71], tannins and phytates [72], α-tocopherol [73], quercetin [74, 75], pterosin [75], ptaquiloside [76], terpene, hopan-triterpene lactone [51], and lutein [77] were also isolated from D. esculentum. Additionally, four phenolic compounds ((2R)-3-(4′-hydroxyphenyl) lactic acid, trans-cinnamic acid, protocatechuic acid, and rutin) and three ecdysteroids (amarasterone A1, makisterone C, and ponasterone A) were isolated from young fronds of D. esculentum collected from Japan [78] while 26 bioactive compounds were identified in the methanolic extracts of young fronds of D. esculentum collected from Indian Himalaya [60]. The major compounds present in the species were identified as pentadecanoic acid, β-sitosterol, neophytadiene, α-linolenic acid, methyl palmitate, diisobutyl phthalate, phytol, and 10,12 hexadecadien-1-ol [60]. These all major compounds are shown in Figures 3(a) and 3(b), respectively.
Figure 3.
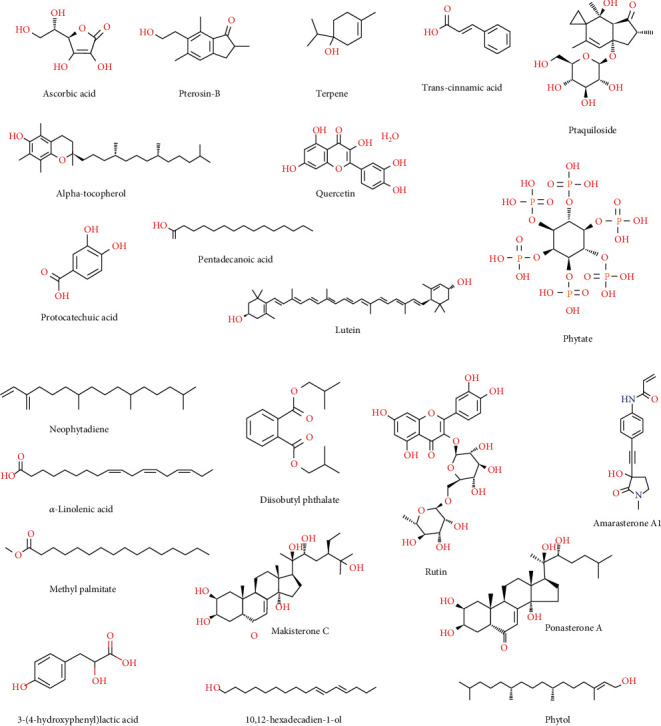
(a) Main nonoil bioactive components of D. esculentum. (b) Main nonoil bioactive components of D. esculentum.
4. Biological Applications
Among the functional properties of D. esculentum, the antioxidant, anti-inflammatory, antimicrobial, antidiabetic, and immune-modulatory activities can be considered as potentially contributing to the preventive and pharmacological values of this plant species (Figure 4). The following sections reviewed the abovementioned functional biological activities of different D. esculentum extracts.
Figure 4.
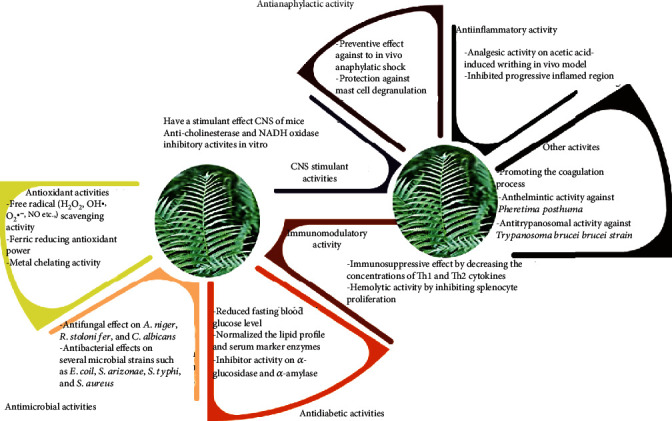
Summary of the proposed biological activities of Diplazium esculentum.
4.1. Antioxidant Activities
The botanicals can be considered as safe and cost-effective natural antioxidants capturing free radicals and may help in the prevention and the treatment of different diseases [79, 80]. Recently, a research group from Indonesia reported that the methanolic extract of D. esculentum showed a good antioxidant activity with an IC50 value of 123.95 ppm according to 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay [2].
In an in vitro study, the nutritional properties and antioxidant capacity of D. esculentum were evaluated on the ethanol extract of the edible parts. The phytochemical analysis indicated that the ethanolic extract possesses significant concentrations of flavonoids (90.6–144.5 mg QE/gm) and tannins (26.8–57.2 mg GAE/gm). Considerable antioxidant activities of D. esculentum were revealed using different antioxidant assays including DPPH radical scavenging (IC50=146.51 μg/mL), superoxide radical scavenging (IC50=111.17 μg/mL), hydroxyl radical scavenging (IC50=43.45 μg/mL), and reducing power (IC50=76.36 μg/mL) assays.
In another study, the antioxidant activity of D. esculentum, extracted by using pressurized hot water extraction (PHWE) method, was reported [81]. The results demonstrated that the optimum condition for the best antioxidant activity of PHWE was at 175°C, 21 min extraction time (2 g dried powder in 50 mL water) in Box-Behnken design. The plant extract showed moderate DPPH scavenging activity (EC50 = 1241.14 μg/mL). The hydro-alcoholic extract of D. esculentum leaf was evaluated for antioxidant activity using the DPPH and nitric oxide assays [82]. The IC50 value of the plant extract for DPPH and NO inhibition activity was found to be 138.8 and 151.9 mg/mL, respectively.
The methanolic extract of D. esculentum fronds showed promising antioxidant activity using different assays (DPPH, ABTS, NO, metal chelating, and superoxide scavenging activity) [64]. The IC50 values of the plant extract was recorded as 3.8, 4.6, 0.59, and 2.24 mg/mL for DPPH, ABTS, metal chelating, and superoxide scavenging activity, respectively, while nitric oxide, hydroxyl ion, and FRAP assays were recorded as 100-10000 μg/mL, 100-10000 μg/mL, and 0.095-0.121 mM Fe2+ equivalent. Table 4 includes detailed information about previous antioxidant activities.
Table 4.
Previous antioxidant studies in Diplazium esculentum.
| Plant part used and solvent system | Name of assay | Key results | References | ||
|---|---|---|---|---|---|
| Plant extracts | Positive control | Antioxidant activity∗ | |||
| Whole plant, (chloroform, n-butanol, aqueous) | Free radical scavenging (DPPH) | IC50 = 287-404 μg/mL | IC50 = 17.45 μg/mL | Moderate | [77] |
| Radical cation scavenging activity (ABTS+) | IC50 = 191-273 μg/mL | IC50 = 08.44 μg/mL | Moderate | ||
| Ferric reducing antioxidant power (FRAP) | 0.44-0.55 mg/g | — | |||
|
| |||||
| Leaves (methanol) | Free radical scavenging (DPPH) | 31.35-57.95% inhibition | 91.99-97.03% inhibition | Moderate | [57] |
|
| |||||
| Leaves (methanol) | Free radical scavenging (DPPH) | IC50 = 402.88 μg/mL | IC50 = 324.86 μg/mL | Weak | [83] |
|
| |||||
| Leaves (protein) | Free radical scavenging (DPPH) | IC50 = 10.23 mg/mL | — | — | [84] |
| Free radical scavenging (DMPD·+) | IC50 = 14.67 mg/mL | — | — | ||
| Radical cation scavenging activity (ABTS+) | IC50 = 07.95 mg/mL | — | — | ||
|
| |||||
| Leaves (not reported) | Free radical scavenging (DPPH) | 336-3359 ORAC unit2/g | — | — | [50] |
|
| |||||
| Leaves (ethanol, vinegar, acetic acid, aqueous) | Free radical scavenging activity (DPPH) | 258-303 μmol TE/100 g | — | — | [85] |
|
| |||||
| Leaves (chloroform, methanol) | Total antioxidant capacities (TAC) | 181.94-207.41 mg/g | — | — | [46] |
| Free radical scavenging (DPPH) | IC50 = 5907-95669 μg/mL | IC50 = 13.76 μg/mL | Weak | ||
|
| |||||
| Leaves (methanol) | Free radical scavenging activity (DPPH) | IC50 = 1.73 mg/mL | — | — | [86] |
| Metal chelating activity | 52.07 mg/mL | — | — | ||
| Ferric reducing antioxidant power (FRAP) | 2.12 μg/mg | — | — | ||
| Radical cation scavenging activity (ABTS+) | IC50 = 0.03 mg/mL | — | — | ||
|
| |||||
| Fronds (aqueous, ethanol) | Radical cation scavenging activity (ABTS+) | 09.60-57.84% inhibition | — | — | [65] |
| Hydrogen peroxide scavenging (H2O2) | 15-40% inhibition | 50% inhibition | Strong | ||
|
| |||||
| Leaves (methanol) | Hydroxyl radical scavenging (OH·) | IC50 = 811.00 μg/mL | IC50 = 571.00 μg/mL | Weak | [87] |
| Superoxide anion scavenging (O2·−) | IC50 = 90.39 μg/mL | IC50 = 42.06 μg/mL | Strong | ||
| Nitric oxide radical scavenging (NO) | IC50 = 204.28 μg/mL | IC50 = 90.82 μg/mL | Moderate | ||
| Hydrogen peroxide scavenging (H2O2) | IC50 = 4.17 mg/mL | IC50 = 3.24 μg/mL | Strong | ||
| Peroxynitrite scavenging (ONOO−) | IC50 = 3.35 mg/mL | IC50 = 0.87 μg/mL | Strong | ||
| Singlet oxygen scavenging (1O2) | IC50 = 278.88 μg/mL | IC50 = 46.15 μg/mL | Moderate | ||
| Hypochlorous acid scavenging (HOCl) | IC50 = 338.96 μg/mL | IC50 = 235.95 μg/mL | Moderate | ||
| Iron chelating | IC50 = 1.33 mg/mL | IC50 = 0.001 μg/mL | Strong | ||
| Lipid peroxidation inhibition | IC50 = 141.67 μg/mL | IC50 = 6.76 μg/mL | Moderate | ||
|
| |||||
| Leaves (petroleum ether, chloroform, acetone, methanol, aqueous) | Ferric reducing antioxidant power (FRAP) | 0.22-7.6 mM/dry weight | — | — | [88] |
|
| |||||
| Leaves (aqueous-methanol, acetone) | Free radical scavenging (DPPH) | IC50 = 0.92-3.60 mg dry wt. | — | — | [89] |
| Ferric reducing antioxidant power (FRAP) | 4.99-8.78 mg/g | — | — | ||
|
| |||||
| Leaves (methanol) | Free radical scavenging (DPPH) | EC50 = 3353.2 μg/mg | EC50 = 322.4 μg/mg | Weak | [90] |
|
| |||||
| Leaves (aqueous) | Free radical scavenging (DPPH) | 50 μmol/g | — | — | [91] |
| Ferric reducing antioxidant power (FRAP) | 100 mol/g | — | — | ||
| Cupric ions chelation assay | 80% inhibition | — | — | ||
(-): not mentioned in the reference papers; (∗): antioxidant activity was considered strong (less than 100 μg/mL), moderate (100 to 500 μg/mL), weak (500 to 1000 μg/mL), or inactive (over 1000 μg/mL) compared with control.
4.2. Antimicrobial Activities
Recently, several pathogenic microorganisms have developed antibiotic resistance, and these antibiotics can have undesirable side effects [92]. Thus, researchers are focusing on botanicals for the development of herbal-based antibiotic substitutes [93]. Table 5 includes antimicrobial studies performed with D. esculentum. Antimicrobial activity was considered good (minimum inhibitory concentration (MIC) less than 100 μg/mL), moderate (MIC from 100 to 500 μg/mL), weak (MIC from 500 to 1000 μg/mL), or inactive (MIC over 1000 μg/mL). Inactive results of antimicrobial activities of D. esculentum did not included in this study [46, 94].
Table 5.
Antimicrobial activities of Diplazium esculentum.
| Plant part used and solvent system | Microorganism | Antimicrobial activity | MIC (μg/mL) | MBC or MFC (μg/mL) | Reference |
|---|---|---|---|---|---|
| Aerial parts (ethanol) |
Bacillus cereus
Escherichia coli Aspergillus ochraceus Bacillus megaterium |
Moderate Moderate Moderate Weak |
200 400 400 800 |
800 >800 800 >800 |
[95] |
|
| |||||
| Aerial parts (ethanol) |
Staphylococcus aureus
Bacillus cereus Klebsiella pneumoniae Pseudomonas aeruginosa Candida albicans Candida parapsilosis Cryptococcus neoformans Issatchenkia orientalis |
Moderate-weak Moderate-weak Moderate-weak Weak Weak-inactive Weak-inactive Moderate Good-moderate |
310-630 310-630 310-630 630 630-1250 1250-2500 310 80-160 |
NA 1250 NA NA 2500 NA 310 160 |
[97] |
| Aerial parts (hexane) |
Cryptococcus neoformans
Issatchenkia orientalis |
Moderate Good |
310 80 |
310 160 |
[97] |
|
| |||||
| Aerial parts (chloroform) |
Staphylococcus aureus
Bacillus cereus Klebsiella pneumoniae Pseudomonas aeruginosa Trichophyton mentagrophytes Candida albicans Cryptococcus neoformans Issatchenkia orientalis |
Moderate-weak Moderate Moderate-weak Weak-inactive Weak-inactive Weak-inactive Moderate Good |
310-630 310 310-630 630-1250 630-1250 630-1250 310 80 |
NA 630 NA NA 1250 NA 310 160 |
[97] |
|
| |||||
| Aerial parts (ethyl acetate) |
Pseudomonas aeruginosa
Cryptococcus neoformans Issatchenkia orientalis |
Weak Moderate Moderate |
630 310 160 |
NA 310 310 |
[97] |
|
| |||||
| Aerial parts (methanol) |
Staphylococcus aureus
Bacillus cereus Klebsiella pneumoniae Pseudomonas aeruginosa Cryptococcus neoformans Issatchenkia orientalis |
Weak-inactive Weak-inactive Weak-inactive Weak-inactive Moderate Moderate |
630-1250 630-1250 630-1250 630-1250 310 160 |
NA 2500 NA NA 310 310 |
[97] |
|
| |||||
| Aerial parts (aqueous) |
Pseudomonas aeruginosa
Cryptococcus neoformans Issatchenkia orientalis |
Weak-inactive Moderate Moderate |
630-1250 160-310 160 |
NA 310 310 |
[97] |
|
| |||||
| Leaves, rhizomes, and roots (aqueous and alcoholic) |
Escherichia coli
Salmonella arizonae Salmonella typhi Staphylococcus aureus |
-∗ -∗ -∗ -∗ |
[96] | ||
|
| |||||
| Leaves (methanol) |
Salmonella paratyphi
Vibrio parahemolyticus Escherichia coli Bacillus megaterium Shigella dysenteriae Shigella boydii |
-∗ -∗ -∗ -∗ -∗ -∗ |
[98] | ||
MBC: minimum bactericidal concentration; MIC: minimum inhibitory concentration; MFC: minimum fungal concentration; NA: no activity; “-”: not tested. Antimicrobial activity was considered good (MIC less than 100 μg/mL), moderate (MIC from 100 to 500 μg/mL), and weak (MIC from 500 to 1000 μg/mL). ∗Only zone inhibition test was performed.
The areal parts of D. esculentum were extracted with ethanol to evaluate the antimicrobial properties by using the disk diffusion method. The crude extract showed considerable antimicrobial activity in terms of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) value. The MIC value was recorded from a range of 200-800 μg/mL (200 μg/mL (Bacillus cereus), 400 μg/mL (Escherichia coli and Aspergillus ochraceus), and 800 μg/mL (Bacillus megaterium)) while MBC from a range of 800 to >800 μg/mL (800 μg/mL (B. cereus, A. ochraceus) and >800 μg/mL (B. megaterium, E. coli)), respectively [95].
Different parts (leaves, rhizomes, and roots) of the D. esculentum were extracted with aqueous and alcoholic solvents to evaluate the antibacterial activity by using the disk diffusion method. Four bacterial strains, namely, E. coli, Salmonella arizonae, Salmonella typhi, and Staphylococcus aureus, were used in this study. The rhizome and root extracts inhibited the growth of microorganisms while leaf extract did not show any inhibition. Additionally, extracts combined with the antibiotic (tetracycline in equal amount) were more potent against bacterial strains than the antibiotic alone [96].
The aerial parts of D. esculentum extracts were evaluated for antimicrobial activity by using a colorimetric broth microdilution method. A total six different solvent extracts (hexane, chloroform, ethyl acetate, ethanol, methanol, and distilled water) were used against a series of microbial strains including S. aureus, B. cereus, Klebsiella pneumoniae, Pseudomonas aeruginosa, E. coli, Acinetobacter baumannii, Candida albicans, Candida parapsilosis, Issatchenkia orientalis, Cryptococcus neoformans, Aspergillus brasiliensis, and Trichophyton mentagrophytes. The plant extract only showed a good-moderate antimicrobial activity against I. orientalis [97].
The methanolic extract of D. esculentum leaves has been evaluated for antibacterial activity by using the disc diffusion method [98]. The plant extract showed slight antibacterial activity (6-10 mm zone of inhibition) against Salmonella paratyphi, Vibrio parahaemolyticus, E. coli, B. megaterium, Shigella dysenteriae, and Shigella boydii among 12 bacterial strains.
The chloroform and methanolic extracts of D. esculentum leaves were evaluated for antimicrobial activity by using the disk diffusion method [46]. The plant extracts showed inactive antimicrobial activity against all the microbial strains tested, namely, K. pneumoniae, S. aureus, E. coli, Salmonella typhimurium, Vibrio cholerae, Sarcina lutea, Bacillus subtilis, and Shigella boydii in terms of MIC (1.6-12.5 mg/mL) value.
The antifungal activity of D. esculentum leaves against three fungal strains using the agar diffusion method has been reported [94]. The methanolic extract showed inactive antifungal activity against Aspergillus niger, Rhizopus stolonifer, and C. albicans in terms of MIC (50-100 mg/mL) and minimum fungal inhibition concentration (100-200 mg/mL).
4.3. Antidiabetic Activities
Diabetes mellitus is a chronic carbohydrate, fat, and protein metabolism disorder characterized by the increase in blood glucose level due to defect of insulin secretion [99]. The inhibition of α-glucosidase and α-amylase enzymes, involved in the digestion of carbohydrates, can significantly reduce the postprandial increase of blood glucose and therefore can be an important strategy in the management of blood glucose level in type 2 diabetic and borderline patients. The antidiabetic activity of D. esculentum through inhibition of α-glucosidase and α-amylase enzymes has been reported [69]. The results demonstrated that D. esculentum extract exhibited the highest α-amylase (92.09%) and α-glucosidase (70.01%) inhibitory activities.
The protective effect of a hydro-alcoholic extract of D. esculentum on streptozocin- (STZ-) induced diabetes was evaluated [82]. In this study, a total of 30 rats were used and treated with plant extract up to 21 days. After the treatment, it was observed that the plant extract (500 mg/kg) reduced (50.2%) the blood glucose level in STZ-induced diabetic rats. Additionally, a significant reduction was recorded in plant extract-treated rats for lipid profiling (p < 0.01), serum marker enzyme activity (p < 0.001), necrosis, and regeneration of beta cells. The plant extract showed dose-dependent activity in all the experiments.
4.4. Immunomodulatory Activity
The immunosuppressive and hemolytic activities of D. esculentum extracts in mouse models have been evaluated [100]. A total of 120 Swiss albino mice (6-8 weeks age) were treated with plant extracts up to 180 days. After this treatment, the plant extract showed significant dose-dependent decreases in body weight, relative spleen weight, number of plaques (formation of antibody secreting cells) formed, hemagglutination antibody titer value, the number of peritoneal macrophages, and the number of cultured splenocytes. The in vitro analysis showed significant dose-dependent increases in the percentage inhibition of splenocyte proliferation as well as the percentage of hemolysis. In other words, the treatment with D. esculentum may act as an immunosuppressive agent.
The impact of boiled D. esculentum on Th1 and Th2 cytokine levels of Swiss albino mice that were treated with different doses of plant extract, daily up to 180 days, has been reported [101]. The outcome of the study demonstrated that the plant extract significantly decreases the concentration of Th1 and Th2 cytokines when compared with controls. In other words, boiled D. esculentum extract may affect some of the innate and cell-mediated immune responses by modulating the level of Th1 and Th2 cytokines.
4.5. CNS Stimulant Activities
The impact of “Ulam” (a fresh Malaysian vegetable, D. esculentum) on cognitive status has been evaluated [102]. In this cross-sectional study, a total of 132 adults were recruited. Socio-demographic information, anthropometric measurements, dietary history, food frequency, and cognitive function were assessed. The average ulam intake by the participants was 15.1 ± 8.2 g/day. The outcome of the study indicated that “pucukpaku” showed protective effects (62.9%) against cognitive decline.
The anticholinesterase and NADH oxidase inhibitory activities of a methanolic extract of D. esculentum have been evaluated [83]. Recently, most of the studies reported that the inhibition of anticholinesterase has been shown to be a strategy for the treatment of neurodegenerative disorders. The results of the study demonstrated that the methanolic extract of D. esculentum inhibited acetyl-cholinesterase and NADH oxidase in a dose-dependent manner, with IC50 values of 272.97 and 265.81 μg/mL.
The CNS stimulant effect of D. esculentum in a mouse model using digital acto-photometer has been reported [88]. The plant water extract showed statistically significant (p < 0.0001) and dose-dependent activity when compared with control and standard caffeine.
4.6. Toxicity Studies (In Vitro and In Vivo)
The methanolic and chloroform extracts of D. esculentum were evaluated for their toxicity using brine shrimp lethality bioassay. Both extracts produced dose-dependent increment in percent mortality of brine shrimp nauplii which indicates the presence of toxic compounds in the extracts. The LC50 values were recorded as 1.87 μg/mL (chloroform), 1.62 μg/mL (methanol), and 0.66 μg/mL (vincristine sulphate as standard drug) [46]. In another study, the toxicity of methanolic extract of D. esculentum using brine shrimp lethality bioassay was reported as significant (LC50 = 18.6 μg/mL). [98]. In other study, the cytotoxicity of ethanolic extract of D. esculentum was evaluated in different cell lines including breast cancer (MDA-MB-231 and MCF-7), colon cancer (Caco-2), liver cancer (HepG2), and normal liver (Chang liver), and no cytotoxic effect was observed [103].
The systemic toxicity and several pathological effects of D. esculentum were evaluated on rabbits and guinea pigs [104]. The study indicated that the plant extract decreased all the pathological functions including growth, body weight, forced motor activity, alterations of blood glucose values, erythrocyte sedimentation rate, mean corpuscular volume, mean corpuscular hemoglobin, total leukocyte count, neutrophil, lymphocyte, and monocyte count, while increased blood SGOT level in both rats and guinea pigs. In other words, the plant extract indicated toxic effects in guinea pigs and rabbits, while rats showed a little adverse effects. Junejo and coworkers reported the nontoxic effects of D. esculentum extract on experimental models and recommended as a potential functional food [67].
The toxicological impact of D. esculentum on male reproductive functions of Swiss albino mice has been reported [105]. A total of 120 male Swiss albino mice of 6-8 weeks of age were fed orally with 80, 160, and 320 mg/kg b.w. of plant material and treated up to 180 days. After this successful treatment, the boiled plant extract showed significant dose and time-dependent decreases in body weight, absolute and relative testis weight, the relative weight of other organs and their biochemical parameters, percentage of live spermatozoa, fertility, and fecundity in plant extract fed mice. In other words, the main outcome of this study is boiled extracts of D. esculentum possess toxic properties that can be slow down the male reproductive functions and may induce infertility.
4.7. Antianaphylactic and Mast Cells Stabilizing Activity
D. esculentum were extracted with aqueous and ethanolic solvents and evaluated for mast cell stability and antianaphylactic activity. In this study, Swiss albino mice (18-20 g) and Wistar rats (150-170 g) were used. A significant reduction was observed in the number of degranulated mast cells of the plant extracts-treated models (p < 0.001). After the administration of both extracts at 250 and 500 mg/kg doses, it showed 72.83%, 76.67%, 69%, and 71.67% intact mast cells. Plant extract demonstrates protective activity against mast cell degranulation. The 500 mg/kg dose of both extracts showed maximum inhibition of the release of myeloperoxidase from lung tissue. Additionally, the plant extract had stabilized the mast cell membrane and decreased the level of nitric oxide in serum and peritoneal fluid [106].
4.8. Anti-inflammatory Activity
The ethanolic extract of D. esculentum was evaluated for anti-inflammatory activity [107]. A total of 25 male mice were recruited in this experiment and divided into 5 groups. The ethanolic extract indicated anti-inflammatory activity on hind paw oedema in terms of inflamed inhibition percent of 125 mg/kg b.w. (71.72%), 250 mg/kg b.w. (81.49%), and 250 mg/kg b.w. (92.60%) in the treated group. In another study, a considerable analgesic activity of D. esculentum was recorded using the acetic acid-induced writhing method in mice [63].
4.9. Other Biological Activities
The aqueous and powder extract of D. esculentum leaves was evaluated for coagulant activity [108]. The plant extracts combined with polyaluminium chloride showed a synergistic effect for all the measured parameters in Kuala Sepetang Landfill Site (KSLS) leachates. The combination was identified as a high molecular weight polymer, and it acted as an anionic coagulant and was also capable of promoting the coagulation process.
Extracts of the rhizome of D. esculentum extracts were evaluated for their anthelmintic activity against Pheretima posthuma. The study included three solvents (ethanol, aqueous, and petroleum ether) and three concentrations (10, 25, and 50 mg/mL), and all the extracts demonstrated significant anthelmintic activity in terms of the time of paralysis and time of death. Ethanolic extract showed the highest activity compared to other solvents, and the activity was recorded in dose-dependent patterns [109].
Silver nanoparticles (10-45 nm) were synthesized using the leaf powder of D. esculentum [110]. The synthesized nanoparticles were evaluated as a catalyst in the degradation of methylene blue and rhodamine B and also evaluated for the anticoagulation activity. The synthesized Ag NPs showed considerable anticoagulation activity. Besides, prominent photocatalytic activity in the degradation of methylene blue and rhodamine B was also recorded.
The antitrypanosomal activity of D. esculentum leaves was evaluated against Trypanosoma brucei brucei strain BS221 [111]. In this study, the ethanolic extract was used with seven different concentrations (0.01 to 12.5 μg/mL), and the extract showed significant antitrypanosomal activity with IC50 value 4.32 μg/mL and a selectivity index (SI) value > 23 in mammalian cell line (Vero, IC50 > 100 μg/mL) when compared with the positive control (pentamidine, IC50 = 4.51 ng/mL).
5. Concluding Remark and Future Prospective
The present manuscript reports traditional uses, nutraceuticals, pharmacognosy, phytochemistry, and pharmacological studies in D. esculentum. The literature survey revealed that D. esculentum is one of the most important and popular wild species of ferns in the Himalaya. It is a widely used species in different traditional systems, but the complete chemical composition and active compounds need to be further elucidated and authenticated by bioassay-guided isolation. However, very limited studies are available for this species, not only in terms of chemical characterization but also in terms of pharmacological evaluation as well. Most of the studies are limited to the in vitro screening and a few for in vivo. Clinical trial studies should be performed to evaluate the safety profile of wild ferns in the human body in terms of antimicrobial activity, antidiabetic activity, anti-inflammatory activity, and immunomodulatory aspects. Apart from this, educating the local people regarding the cultivation, conservation, and sustainable utilization of this plant will help for improving the population size of the species.
Acknowledgments
Help and support received from the Graphic Era University, Dehradun, Uttarakhand, India, is duly acknowledged. P.S. thank Dr. Ashish Bahuguna for their help in drawing the chemical structure of bioactive compounds.
Contributor Information
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
William C. Cho, Email: chocs@ha.org.hk.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declared that they have no conflict of interest regarding this manuscript.
References
- 1.Alonso-Amelot M. E., Oliveros A., Calcagno-Pisarelli M. P. Phenolics and condensed tannins in relation to altitude in neotropical Pteridium spp: a field study in the Venezuelan Andes. Biochemical Systematics and Ecology. 2004;32(11):969–981. doi: 10.1016/j.bse.2004.03.005. [DOI] [Google Scholar]
- 2.Amna U., Mardina V. Antioxidant activity of methanol extract of Diplazium esculentum (Retz.) Sw. leaves collected from Aceh. IOP Conference Series: Materials Science and Engineering, Volume 725, 3rd Nommensen International Conference on Technology and Engineering 2019 (3rd NICTE); July 2019; Indonesia. p. p. 012082. [DOI] [Google Scholar]
- 3.Semwal P., Painuli S., Tewari D., Bussmann R. W., Palni L. M. S., Thapliyal A. Assesment of non-timber Brahma Kamal (Saussurea obvallata (DC.) Edgew.), an important Himalayan medicinal plant: Ethnomedicinal, phytochemical and pharmacological overview. Ethnobotany Research & Applications. 2020;19(40) doi: 10.32859/era.19.40.1-15. [DOI] [Google Scholar]
- 4.Zlatic N. M., Stankovic M. S. Variability of secondary metabolites of the species Cichorium intybus L. from different habitats. Plants. 2017;6(3):p. 38. doi: 10.3390/plants6030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharucha Z., Pretty J. The roles and values of wild foods in agricultural systems. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2010;365(1554):2913–2926. doi: 10.1098/rstb.2010.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maikhuri R. K., Rao K. S., Saxena K. G. Bioprospecting of wild edibles for rural development in the central Himalayan mountains of India. Mountain Research and Development. 2004;24(2):110–113. doi: 10.1659/0276-4741(2004)024[0110:BOWEFR]2.0.CO;2. [DOI] [Google Scholar]
- 7.Sharma M., Dwivedi P., Singh Rawat A., Dwivedi A. K. Nutrition nutraceuticals: a proactive approach for healthcare. Nutraceuticals. 2016;4:79–116. doi: 10.1016/B978-0-12-804305-9.00003-8. [DOI] [Google Scholar]
- 8.Konsam S., Thongam B., Handique A. K. Assessment of wild leafy vegetables traditionally consumed by the ethnic communities of Manipur, northeast India. Journal of Ethnobiology and Ethnomedicine. 2016;12(1):p. 9. doi: 10.1186/s13002-016-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belwal T., Pandey A., Bhatt I. D., Rawal R. S., Luo Z. Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Scientific Reports. 2019;9(1):p. 5894. doi: 10.1038/s41598-019-42270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra S., Maikhuri R., Dhyani D., Rao K. Assessment of traditional rights, local interference and natural resource management in Kedarnath Wildlife Sanctuary. International Journal of Sustainable Development & World Ecology. 2009;16(6):404–416. doi: 10.1080/13504500903332008. [DOI] [Google Scholar]
- 11.PPG I. A community-derived classification for extant lycophytes and ferns. Journal of Systematics and Evolution. 2016;54(6):563–603. doi: 10.1111/jse.12229. [DOI] [Google Scholar]
- 12.Manickam V., Irudayaraj V. Pteridophyte flora of the Western Ghats. South India: BI publications; 1992. [Google Scholar]
- 13.Manickam V., Irudyaraj V. Pteridophytic flora of Nilgiris South India. Dehradun: Bishen Singh Mahendra Pal Singh; 2003. [Google Scholar]
- 14.Quattrocchi U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology (5 Volume Set) CRC press; 2016. [Google Scholar]
- 15.Abe R., Ohtani K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. Journal of Ethnopharmacology. 2013;145(2):554–565. doi: 10.1016/j.jep.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Essien E., Ascrizzi R., Flamini G. Characterization of volatile compounds of Diplazium esculentum. Chemistry of Natural Compounds. 2019;55(5):958–959. doi: 10.1007/s10600-019-02860-y. [DOI] [Google Scholar]
- 17.Kadir M. F., Sayeed M. S. B., Setu N. I., Mostafa A., Mia M. Ethnopharmacological survey of medicinal plants used by traditional health practitioners in Thanchi, Bandarban Hill Tracts, Bangladesh. Journal of Ethnopharmacology. 2014;155(1):495–508. doi: 10.1016/j.jep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 18.Lense O. Biological screening of selected traditional medicinal plants species utilized by local people of Manokwari, West Papua Province. Nusantara Bioscience. 2016;3(3) doi: 10.13057/nusbiosci/n030306. [DOI] [Google Scholar]
- 19.Roosita K., Kusharto C. M., Sekiyama M., Fachrurozi Y., Ohtsuka R. Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. Journal of Ethnopharmacology. 2008;115(1):72–81. doi: 10.1016/j.jep.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Tag H., Kalita P., Dwivedi P., Das A., Namsa N. D. Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, northeast, India. Journal of Ethnopharmacology. 2012;141(3):786–795. doi: 10.1016/j.jep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Zannah F., Amin M., Suwono H., Lukiati B. Phytochemical screening of Diplazium esculentum as medicinal plant from Central Kalimantan, Indonesia. AIP Conference Proceedings. 2017;1844, article 050001 doi: 10.1063/1.4983439. [DOI] [Google Scholar]
- 22.Sarkar B., Basak M., Chowdhury M., Das A. Importance of Diplazium esculentum (Retz.) Sw. (Athyriaceae) on the lives of local ethnic communities in Terai and Duars of West Bengal-A report. Plant Archives. 2018;18:439–442. [Google Scholar]
- 23.Kutum A., Sarmah R., Hazarika D. An ethnobotanical study of Mishing tribe living in fringe villages of Kaziranga National Park of Assam, India. Indian Journal of Fundamental and Applied Life Sciences. 2011;1:45–61. [Google Scholar]
- 24.Sousa E. C., Uchôa-Thomaz A. M. A., Carioca J. O. B., et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Science and Technology. 2014;34(1):135–142. doi: 10.1590/S0101-20612014000100020. [DOI] [Google Scholar]
- 25.Archana G. N., Pradeesh S., Chinmayee M. D., Mini I., Swapna T. Prospects in Bioscience: Addressing the issues. Springer; 2012. Diplazium esculentum: a wild nutrient-rich leafy vegetable from Western Ghats; pp. 293–301. [DOI] [Google Scholar]
- 26.Chettri S., Manivannan S., Muddarsu V. R. Nutrient and elemental composition of wild edible ferns of the Himalaya. American fern journal. 2018;108(3):95–106. doi: 10.1640/0002-8444-108.3.95. [DOI] [Google Scholar]
- 27.Irawan D., Wijaya C. H., Limin S. H., Hashidoko Y., Osaki M., Kulu I. P. Ethnobotanical study and nutrient potency of local traditional vegetables in Central Kalimantan. Tropics. 2006;15(4):441–448. doi: 10.3759/tropics.15.441. [DOI] [Google Scholar]
- 28.Jasim H. S., Idris M., Abdullah A., Kadhum A. Determination of heavy metals in soil and different parts of Diplazium esculentum (medicinal fern) AIP Conference Proceedings. 2014;1614:713–718. doi: 10.1063/1.4895289. [DOI] [Google Scholar]
- 29.Koniyo Y., Lumenta C., Olii A. H., Mantiri R. O. The characteristic and nutrients concentrated leaves of vegetable fern (Diplazium esculentum (Retz.) Swartz) live in dofferent locations. Journal of Physics: Conference Series. 2019;1387, article 012003 doi: 10.1088/1742-6596/1387/1/012003. [DOI] [Google Scholar]
- 30.Ogle M., Dao H. T. A., Mulokozi G., Hambraeus L. Micronutrient composition and nutritional importance of gathered vegetables in Vietnam. International Journal of Food Sciences and Nutrition. 2001;52(6):485–499. doi: 10.1080/713671806. [DOI] [PubMed] [Google Scholar]
- 31.Della R. H., Wijaya C. H., Hashidoko Y., et al. Concentration of some trace elements in two wild edible ferns, Diplazium esculentum and Stenochlaena palutris, inhabiting tropical peatlands under different environments in Central Kalimantan. Eurasian Journal of Forest Research. 2017;20:11–20. [Google Scholar]
- 32.Sayeed A., Islam M. S., Uddin M. N., et al. Nutritional status of exotic and indigenous vegetables. International Journal of Vegetable Science. 2020;27(1):86–95. doi: 10.1080/19315260.2020.1713957. [DOI] [Google Scholar]
- 33.Tongco J. V. V., Villaber R. A. P., Aguda R. M., Razal R. A. Nutritional and phytochemical screening, and total phenolic and flavonoid content of Diplazium esculentum (Retz.) Sw. from Philippines. Journal of Chemical and Pharmaceutical Research. 2014;6:238–242. [Google Scholar]
- 34.Zihad S. N. K., Gupt Y., Uddin S. J., et al. Nutritional value, micronutrient and antioxidant capacity of some green leafy vegetables commonly used by southern coastal people of Bangladesh. Heliyon. 2019;5(11, article e02768) doi: 10.1016/j.heliyon.2019.e02768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koniyo Y., Lumenta C., Olii A., Mantiri R., Pasisingi N. Nutrition of local wild edible fern (Diplazium esculentum) leaves. IOP Conference Series: Earth and Environmental Science. 2021;637, article 012008 doi: 10.1088/1755-1315/637/1/012008. [DOI] [Google Scholar]
- 36.Neamsuvan O., Ruangrit T. A survey of herbal weeds that are used to treat gastrointestinal disorders from southern Thailand: Krabi and Songkhla provinces. Journal of Ethnopharmacology. 2017;209:318–327. doi: 10.1016/j.jep.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 37.Rahman M. M., Masum G. Z. H., Sharkar P., Sima S. N. Medicinal plant usage by traditional medical practitioners of rural villages in Chuadanga district, Bangladesh. International Journal of Biodiversity Science, Ecosystem Services & Management. 2013;9(4):330–338. doi: 10.1080/21513732.2013.841757. [DOI] [Google Scholar]
- 38.Shil S., Choudhury M. D. Ethnomedicinal importance of pteridophytes used by Reang tribe of Tripura, North East India. Ethnobotanical Leaflets. 2009;2009:p. 10. [Google Scholar]
- 39.Pegu R., Gogoi J., Tamuli A. K., Teron R. Ethnobotanical study of wild edible plants in Poba Reserved Forest, Assam, India: multiple functions and implications for conservation. Research Journal of Agriculture and Forestry Sciences. 2013;2320, article 6063 [Google Scholar]
- 40.Kunkel G. Plants for Human Consumption. Koenigstein: Koeltz Scientific Books; 1984. [Google Scholar]
- 41.Chandra K., Nautiyal B., Nautiyal M. Ethno-botanical resources as supplementary foods and less known wild edible fruits in district Rudraprayag, Uttarakhand, India. Journal of Human Ecology. 2013;42(3):259–271. doi: 10.1080/09709274.2013.11906600. [DOI] [Google Scholar]
- 42.Das A. K., Dutta B., Sharma G. Medicinal plants used by different tribes of Cachar district, Assam. Indian Journal of Traditional Knowledge. 2008;7:446–454. [Google Scholar]
- 43.Farooquee N. A., Majila B., Kala C. Indigenous knowledge systems and sustainable management of natural resources in a high altitude society in Kumaun Himalaya, India. Journal of Human Ecology. 2004;16(1):33–42. doi: 10.1080/09709274.2004.11905713. [DOI] [Google Scholar]
- 44.Sen A., Ghosh P. A note on the ethnobotanical studies of some pteridophytes in Assam. Indian Journal of Traditional Knowledge. 2011;10:292–295. [Google Scholar]
- 45.Yumkham S., Singh P. Less known ferns and fern-allies of Manipur with ethnobotanic uses. Indian Journal of Traditional Knowledge. 2011;10:287–291. [Google Scholar]
- 46.Akter S., Hossain M. M., Ara I., Akhtar P. Investigation of in vitro antioxidant, antimicrobial and cytotoxic activity of Diplazium esculentum (Retz.) Sw. International Journal of Advances in Pharmacy, Biology and Chemistry. 2014;3:723–733. [Google Scholar]
- 47.Kagyung R., Gajurel P., Rethy P., Singh B. Ethnomedicinal plants used for gastro-intestinal diseases by Adi tribes of Dehang-Debang Biosphere Reserve in Arunachal Pradesh. Indian Journal of Traditional Knowledge. 2010;9:496–501. [Google Scholar]
- 48.Nwosu M. O. Ethnobotanical studies on some pteridophytes of Southern Nigeria. Economic Botany. 2002;56(3):255–259. doi: 10.1663/0013-0001(2002)056[0255:ESOSPO]2.0.CO;2. [DOI] [Google Scholar]
- 49.Uprety Y., Boon E. K., Poudel R. C., et al. Non-timber forest products in Bardiya district of Nepal: indigenous use, trade and conservation. Journal of Human Ecology. 2010;30(3):143–158. doi: 10.1080/09709274.2010.11906283. [DOI] [Google Scholar]
- 50.Amoroso V. B., Lagumbay A., Mendez R. A., Dela Cruz R. Y., Villalobos A. P. Bioactives in three Philippine edible ferns. Asia Life Sciences. 2014;23:445–454. [Google Scholar]
- 51.Astuti M. D., Kuntorini E. M., Wisuda F. E. P. Isolasi dan identifikasi terpenoid dari fraksi n-butanol herba lampasau (Diplazium esculentum Swartz) Jurnal Kimia Valensi. 2014;4 doi: 10.15408/jkv.v4i1.1048. [DOI] [Google Scholar]
- 52.Bhatia H., Sharma Y. P., Manhas R., Kumar K. Traditionally used wild edible plants of district Udhampur, J&K, India. Journal of Ethnobiology and Ethnomedicine. 2018;14(1):p. 73. doi: 10.1186/s13002-018-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thakur A., Singh S., Puri S. Exploration of wild edible plants used as food by Gaddis-a tribal community of the Western Himalaya. The Scientific World Journal. 2020;2020:6. doi: 10.1155/2020/6280153.6280153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rout S., Panda T., Mishra N. Ethnomedicinal studies on some pteridophytes of similipal biosphere reserve, Orissa, India. International Journal of Medicine and Medical Sciences. 2009;1:192–197. [Google Scholar]
- 55.Jahan R., Jannat K., Shoma J. F., Khan M. A., Shekhar H. U., Rahmatullah M. Herbal Medicine in India. Springer; 2020. Drug discovery and herbal drug development: a special focus on the anti-diarrheal plants of Bangladesh; pp. 363–400. [DOI] [Google Scholar]
- 56.Rai M. Ethno-medical studies of patalkot and tamiya (distt. Chhindwara) MP–plants used as tonic. Ancient Science of Life. 1987;7:p. 119. [PMC free article] [PubMed] [Google Scholar]
- 57.Pradhan S., Manivannan S., Tamang J. P. Proximate, mineral composition and antioxidant properties of some wild leafy vegetables. Journal of Scientific and Industrial Research. 2015;74:155–159. [Google Scholar]
- 58.Shrestha S. Use and chemical analysis of wild food in protected areas of Nepal. Nepalese Journal of Agricultural Sciences. 2011;9:103–106. [Google Scholar]
- 59.Rai A. K., Sharma R. M., Tamang J. P. Food value of common edible wild plants of Sikkim. Journal of Hill Research. 2005;18:99–103. [Google Scholar]
- 60.Naik B., Maurya V. K., Kumar V., Kumar V., Upadhyay S., Gupta S. Phytochemical analysis of Diplazium esculentum reveals the presence of medically important components. Current Nutrition & Food Science. 2021;17(2):210–215. doi: 10.2174/1573401316999200614162834. [DOI] [Google Scholar]
- 61.Ghanbari R., Anwar F., Alkharfy K. M., Gilani A.-H., Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—a review. International Journal of Molecular Sciences. 2012;13(3):3291–3340. doi: 10.3390/ijms13033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muhammad G., Hussain M. A., Jantan I., Bukhari S. N. A. Mimosa pudica L., a high-value medicinal plant as a source of bioactives for pharmaceuticals. Comprehensive Reviews in Food Science and Food Safety. 2016;15(2):303–315. doi: 10.1111/1541-4337.12184. [DOI] [PubMed] [Google Scholar]
- 63.Chawla S., Chawla S., Ram V., Semwal A., Singh R. Analgesic activity of medicinally important leaf of Diplazium esculentum. African Journal of Pharmacy and Pharmacology. 2015;9:628–632. doi: 10.5897/ajpp2015.4316. [DOI] [Google Scholar]
- 64.Choudhury J., Majumdar S., Roy S., Chakraborty U. Antioxidant activity and phytochemical screening of two edible wetland pteridophytes Diplazium esculentum (Retz) Sw and Marsilea minuta L.–a comparative study. World Journal of Pharmaceutical and Medical Research. 2017;3:195–203. [Google Scholar]
- 65.Das B., Paul T., Apte K. G., Chauhan R., Saxena R. C. Evaluation of antioxidant potential & quantification of polyphenols of Diplazium esculentum Retz. with emphasis on its HPTLC chromatography. Journal of Pharmacy Research. 2013;6(1):93–100. doi: 10.1016/j.jopr.2012.11.020. [DOI] [Google Scholar]
- 66.Halimatussakdiah H., Amna U., Wahyuningsih P. Preliminary phytochemical analysis and larvicidal activity of edible fern (Diplazium esculentum (retz.) sw.) extract against culex. Jurnal Natural. 2018;18(3):141–147. doi: 10.24815/jn.v0i0.11335. [DOI] [Google Scholar]
- 67.Junejo J. A., Ghoshal A., Mondal P., et al. In-vivo toxicity evaluation and phytochemical, physicochemical analysis of Diplazium esculentum (Retz.) Sw. leaves a traditionally used North-Eastern Indian vegetable. Advances in Bioresearch. 2015;6(5) [Google Scholar]
- 68.Khatoniar S., Barooah M. S., Baruah I. C. Qualitative and quantitative phytochemical assessment and antioxidant activity of selected green leafy vegetables of Assam. Journal of Pharmacognosy and Phytochemistry. 2018;7:1762–1765. [Google Scholar]
- 69.Tabiano J., Deliman Y. In vitro inhibitory activity of Atuna racemosa, Euphorbia hirta and Diplazium esculentum juices against α-amylase and α-glucosidase. International Seminar on Science and Technology. 2014;2014:p. 89. [Google Scholar]
- 70.Caldwell M. Ascorbic acid content of Malaysian leaf vegetables. Ecology of Food and Nutrition. 1972;1(4):313–317. doi: 10.1080/03670244.1972.9990303. [DOI] [Google Scholar]
- 71.Srivastava S., Srivastava S., Saksena V., Nigam S. A flavanone glycoside from Diplazium esculentum. Phytochemistry. 1981;20(4):p. 862. doi: 10.1016/0031-9422(81)85203-X. [DOI] [Google Scholar]
- 72.Hayati I. N., Aminah A., Mamot S., Aini I. N., Lida H. M. N. Physical characteristics of modified milkfat in high-melting fat preparation. International Journal of Food Sciences and Nutrition. 2002;53(1):43–54. doi: 10.1080/09637480120057000. [DOI] [PubMed] [Google Scholar]
- 73.Ching L. S., Mohamed S. Alpha-tocopherol content in 62 edible tropical plants. Journal of Agricultural and Food Chemistry. 2001;49(6):3101–3105. doi: 10.1021/jf000891u. [DOI] [PubMed] [Google Scholar]
- 74.Miean K. H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Journal of Agricultural and Food Chemistry. 2001;49(6):3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 75.Pathania S., Kumar P., Singh S., et al. Detection of ptaquiloside and quercetin in certain Indian ferns. Current Science. 2012;102(12):1683–1691. [Google Scholar]
- 76.Somvanshi R., Lauren D., Smith B., et al. Estimation of the fern toxin, ptaquiloside, in certain Indian ferns other than bracken. Current Science. 2006;102(12):1547–1552. [Google Scholar]
- 77.Wali A., Sharma S., Walia M., et al. Two edible ferns of Western Himalaya: a comparative in vitro nutritional assessment, antioxidant capacity and quantification of lutein by UPLC-DAD. International Journal of Food and Nutritional Sciences. 2016;5:p. 9. [Google Scholar]
- 78.Watanabe M., Miyashita T., Devkota H. P. Phenolic compounds and ecdysteroids of Diplazium esculentum (Retz.) Sw. (Athyriaceae) from Japan and their chemotaxonomic significance. Biochemical Systematics and Ecology. 2021;94, article 104211 doi: 10.1016/j.bse.2020.104211. [DOI] [Google Scholar]
- 79.Kumar Y., Yadav D. N., Ahmad T., Narsaiah K. Recent trends in the use of natural antioxidants for meat and meat products. Comprehensive Reviews in Food Science and Food Safety. 2015;14(6):796–812. doi: 10.1111/1541-4337.12156. [DOI] [Google Scholar]
- 80.Maqsood S., Benjakul S., Abushelaibi A., Alam A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: a detailed review. Comprehensive Reviews in Food Science and Food Safety. 2014;13(6):1125–1140. doi: 10.1111/1541-4337.12106. [DOI] [Google Scholar]
- 81.Nur A., Khairul F., Nuradibah M., Noor S. Optimization of Diplazium esculentum extract using pressurized hot water extractor by Box-Behnken design of experiments and its antioxidative behavior. IOP Conference Series: Materials Science and Engineering. 2018;429, article 012064 doi: 10.1088/1757-899x/429/1/012064. [DOI] [Google Scholar]
- 82.Junejo J. G., Gogoi G., Islam J., et al. Exploration of antioxidant, antidiabetic and hepatoprotective activity of Diplazium esculentum - A wild edible plant from North Eastern India. Future Journal of Pharmaceutical Sciences. 2018;4(1):93–101. doi: 10.1016/j.fjps.2017.10.005. [DOI] [Google Scholar]
- 83.Roy S., Dutta S., Chaudhuri T. K. In vitro assessment of anticholinesterase and NADH oxidase inhibitory activities of an edible fern, Diplazium esculentum. Journal of Basic and Clinical Physiology and Pharmacology. 2015;26(4):395–401. doi: 10.1515/jbcpp-2014-0100. [DOI] [PubMed] [Google Scholar]
- 84.Rana V., Bachheti R., Gogoi S., Gupta P., Joshi G. Physicochemical, functional and antioxidant properties of Diplazium esculentum leaf protein concentrate. Current Traditional Medicine. 2015;1(2):145–158. doi: 10.2174/221508380102151029152654. [DOI] [Google Scholar]
- 85.Jayag E. P., Acabal A. D. In vitro free radical scavenging activity of Bago (Gnetum gnemon Linn.), Pako (Diplazium esculentum (Retz.) Sw.) and Saluyot (Corchorus olitorius Linn.) leaf extracts. Journal of Society and Technology. 2014;4:17–24. [Google Scholar]
- 86.Gupta S., Ghosal M., Biswas R., Saha B., Das A., Mandal P. Evaluation of in vitro antioxidant activity of methanolic extracts of some ferns from Mawsynram of Meghalaya, India. International Journal of Current Science. 2014;12:E87–E97. [Google Scholar]
- 87.Roy S., Hazra B., Mandal N., Chaudhuri T. K. Assessment of the antioxidant and free radical scavenging activities of methanolic extract of Diplazium esculentum. International Journal of Food Properties. 2013;16(6):1351–1370. doi: 10.1080/10942912.2011.587382. [DOI] [Google Scholar]
- 88.Kaushik A., Jijta C., Kaushik J. J., Zeray R., Ambesajir A., Beyene L. FRAP (Ferric reducing ability of plasma) assay and effect of Diplazium esculentum (Retz) Sw.(a green vegetable of North India) on central nervous system. Indian Journal of Natural Products and Resources. 2012;3:228–231. [Google Scholar]
- 89.Seal T. Antioxidant activity of some wild edible plants of Meghalaya state of India: a comparison using two solvent extraction systems. International Journal of Nutrition and Metabolism. 2012;4:51–56. doi: 10.5897/IJNAM11.060. [DOI] [Google Scholar]
- 90.Nanasombat S., Teckchuen N. Antimicrobial, antioxidant and anticancer activities of Thai local vegetables. Journal of Medicinal Plants Research. 2009;3:443–449. [Google Scholar]
- 91.Wong S., Leong L., Williamkoh J. Antioxidant activities of aqueous extracts of selected plants. Food Chemistry. 2006;99(4):775–783. doi: 10.1016/j.foodchem.2005.07.058. [DOI] [Google Scholar]
- 92.Sheriff Z. Nature Pharmacy Series Vol. 1. Spectrum Book Limited, Ibadan. UK: Nigeria in Association with Safari Books (Export) Limited; 2001. Modern Herbal Therapy for Common Ailments; pp. 9–84. [Google Scholar]
- 93.Rios J. L., Recio M. C. Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology. 2005;100(1-2):80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 94.Zakaria Z., Sanduran S., Sreenivasan S. Antifungal activity of the edible ferns: application for public health. International Journal of the Humanities. 2010;8(8):113–118. [Google Scholar]
- 95.Mackeen M., Ali A., El-Sharkawy S., et al. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables (ulam) International Journal of Pharmacognosy. 1997;35(3):174–178. doi: 10.1076/phbi.35.3.174.13294. [DOI] [Google Scholar]
- 96.Amit S., Sunil K., Bhatt S., Arvind N. Antibacterial activity of Diplazium esculentum (Retz.) Sw. Pharmacognosy Journal. 2011;3(21):77–79. doi: 10.5530/pj.2011.21.14. [DOI] [Google Scholar]
- 97.Shing G. P., Wen C. L., Wei T. S., Chooi O. H., Soo K. K., Weng S. N. Antifungal and antibacterial properties of three medicinal plants from Malaysia. Pharmacognosy Communications. 2013;3(2):75–81. doi: 10.5530/pc.2013.2.15. [DOI] [Google Scholar]
- 98.Ullah M. O., Haque M., Urmi K. F., et al. Anti-bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pacific Journal of Tropical Biomedicine. 2013;3(1):1–7. doi: 10.1016/S2221-1691(13)60015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irudayaraj S. S., Sunil C., Duraipandiyan V., Ignacimuthu S. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) Lam. leaves in streptozotocin induced diabetic rats. Journal of Ethnopharmacology. 2012;143(2):515–523. doi: 10.1016/j.jep.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Roy S., Tamang S., Dey P., Chaudhuri T. K. Assessment of the immunosuppressive and hemolytic activities of an edible fern, Diplazium esculentum. Immunopharmacology and Immunotoxicology. 2013;35(3):365–372. doi: 10.3109/08923973.2013.775588. [DOI] [PubMed] [Google Scholar]
- 101.Roy S., Chaudhuri T. K. Assessment of Th1 and Th2 cytokine modulatory activity of an edible fern, Diplazium esculentum. Food and Agricultural Immunology. 2015;26(5):690–702. doi: 10.1080/09540105.2015.1007449. [DOI] [Google Scholar]
- 102.You Y. X., Shahar S., Haron H., Yahya H. M., Din N. C. Relationship between traditional Malaysian vegetables (Ulam) intake and cognitive status among middle-aged adults from low cost residential areas. Jurnal Sains Kesihatan Malaysia (Malaysian Journal of Health Sciences) 2020;17 [Google Scholar]
- 103.Rahmat A., Kumar V., Fong L. M., Endrini S., Sani H. A. Determination of total antioxidant activity in three types of local vegetables shoots and the cytotoxic effect of their ethanolic extracts against different cancer cell lines. Asia Pacific Journal of Clinical Nutrition. 2003;12(3):292–295. [PubMed] [Google Scholar]
- 104.Kumar G. N. Studies on pathological effects of linguda (Diplazium esculentum, Retz.) in laboratory rats and guinea pigs. Indian Journal of Veterinary Pathology. 2004;28:p. 149. [Google Scholar]
- 105.Roy S., Chaudhuri T. K. Toxicological assessment of Diplazium esculentum on the reproductive functions of male Swiss albino mouse. Drug and Chemical Toxicology. 2017;40(2):171–182. doi: 10.1080/01480545.2016.1190739. [DOI] [PubMed] [Google Scholar]
- 106.Das B., Paul T., Apte K., Parab P., Chauhan R., Saxena R. Antianaphylactic and mast cell stabilizing activity of Diplazium esculentum Retz. on sensitized wistar rats. Inventi Impact Ethnopharmacol. 2012;3:136–141. [Google Scholar]
- 107.Zaini M., Biworo A., Anwar K. Uji efek antiinflamasi ekstrak etanol herba lampasau (Diplazium esculentum Swartz) terhadap mencit jantan yang diinduksi karagenin-Λ. Jurnal Pharmascience. 2017;3 [Google Scholar]
- 108.Zainol N. A., Aziz H. A., Lutpi N. A. Diplazium esculentum leaf extract as coagulant aid in leachate treatment. In Proceedings of AIP Conference Proceedings. 2017;1835(1, article 020034) [Google Scholar]
- 109.Semwal A. S. MF In-vitro anthelmintic activity of Diplazium esculentum (Retz.) Swiss rhizome extract. Journal of Pharmacognosy and Phytochemistry. 2012;1:84–87. [Google Scholar]
- 110.Paul B., Bhuyan B., Purkayastha D. D., Dhar S. S. Green synthesis of silver nanoparticles using dried biomass of Diplazium esculentum (retz.) sw. and studies of their photocatalytic and anticoagulative activities. Journal of Molecular Liquids. 2015;212:813–817. doi: 10.1016/j.molliq.2015.10.032. [DOI] [Google Scholar]
- 111.Norhayati I., Getha K., Haffiz J. M., et al. In vitro antitrypanosomal activity of Malaysian plants. Journal of Tropical Forest Science. 2013;25(1):52–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


