Fig. 2. To establish “ground-truth” we analyzed 15 authentic patient samples using two established commercial tests.
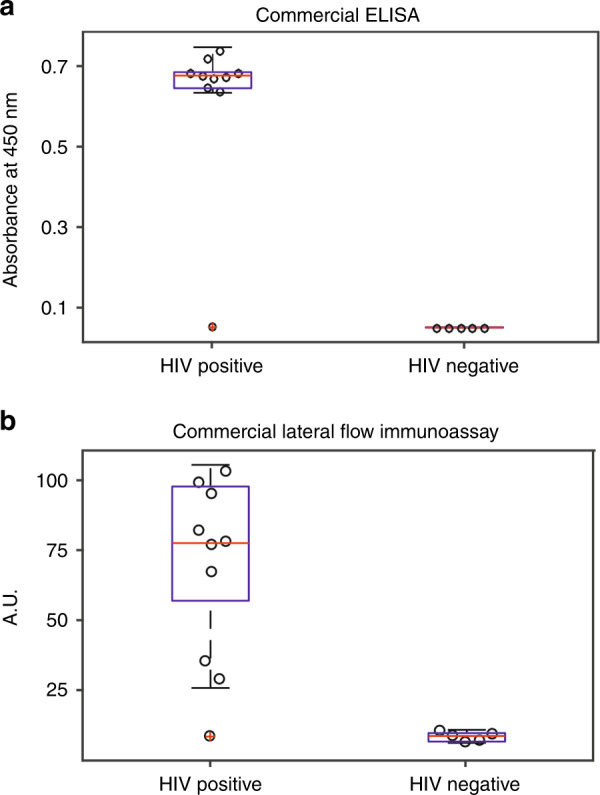
We tested ten (reportedly) HIV-positive and five (reportedly) HIV-negative commercially sourced human serum samples using a a commercial ELISA and b a commercial lateral flow immunoassay. Both achieved 90% clinical sensitivity (both flagged the same reportedly HIV-positive sample as HIV-negative—red dot) and 100% clinical specificity (both correctly determined all five HIV-negative samples to be negative). We quantified the ELISA results measuring the absorption at a wavelength of 450 nm, while lateral flow immunoassays using the “Analyze/Plot Profile” function in the ImageJ Program16.
