Fig. 4. When challenged against our test-bed sample set E-AB sensors achieve the same clinical sensitivity as the commercial tests.
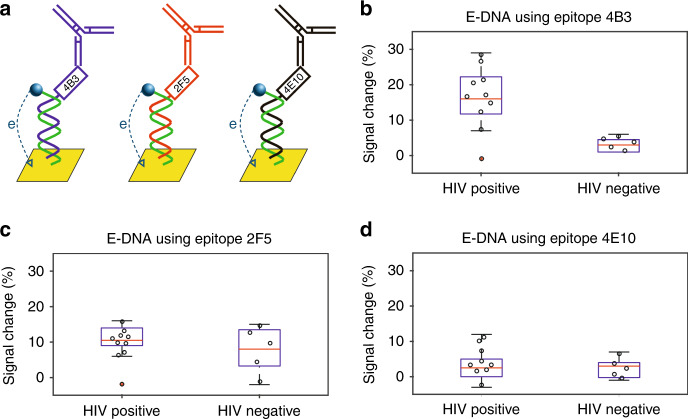
To test the clinical performance of E-DNA scaffold sensors we challenged sensors presenting a high, low, and no immunogenicity gp41 epitopes against patient samples. Ten of these commercially sourced samples were putatively HIV-positive and five negative. b Consistent with the high immunogenicity of the 4B3 epitope, sensors presenting it correctly detected nine out of ten putatively HIV-positive samples (again the missing patient is the same flagged negative by commercial ELISA) and five out of five HIV-negative ones. As was true for the equivalent ELISA and commercial tests, one putatively positive sample did not cause any significant signal change. c Sensors presenting the weakly immunogenic 2F5 epitope, in contrast, failed to flag any of the ten putatively HIV-positive samples as positive (at this serum dilution; see below), while flagging as negative all five of the HIV-negative samples. d Finally, a sensor presenting the effectively nonimmunogenic 4E10 epitope did not respond to any of the 15 samples.