Table 1.
Optimization and control studies.
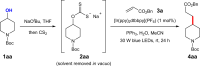 | ||
|---|---|---|
| Entry | Variation from optimized conditions | Yield (%)a |
| 1 | None | 92 (89)b |
| 2 | P(OEt)3 instead of PPh3 | 84 |
| 3 | [Ir(dFCF3ppy)2dtbbpy)](PF6) (1 mol %) | 83 |
| 4 | Na2CO3 instead of NaOtBu | 0 |
| 5 | No CS2 | 0 |
| 6 | No PPh3 | 0 |
| 7 | No photocatalyst | 0 |
| 8 | No light | 0 |
| 9 | Without H2O | 26 |
| 10 | 3a (1.0 equiv) | 66 |
| 11 | PPh3 (0.20 equiv) | 20 |
| 12 | PPh3 (0.20 equiv) + reductants (2.0 equiv) | <20 |
Standard procedure: 1aa (0.20 mmol), NaOtBu (1.0 equiv), THF (1.2 mL), CS2 (1.5 equiv), 3 h. After removing solvent in vacuo, then 3a (2.0 equiv), [Ir(ppy)2dtbbpy](PF6) (1 mol%), PPh3 (1.2 equiv), H2O (7.0 equiv), MeCN (2.0 mL), 24 h blue LED irradiation.
Boc t-butoxycarbonyl.
aDetermined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard.
bIsolated yield.
