Figure 5. Poly(I:C) induces NETs probably by increasing PAD4 expression and its interaction with histones.
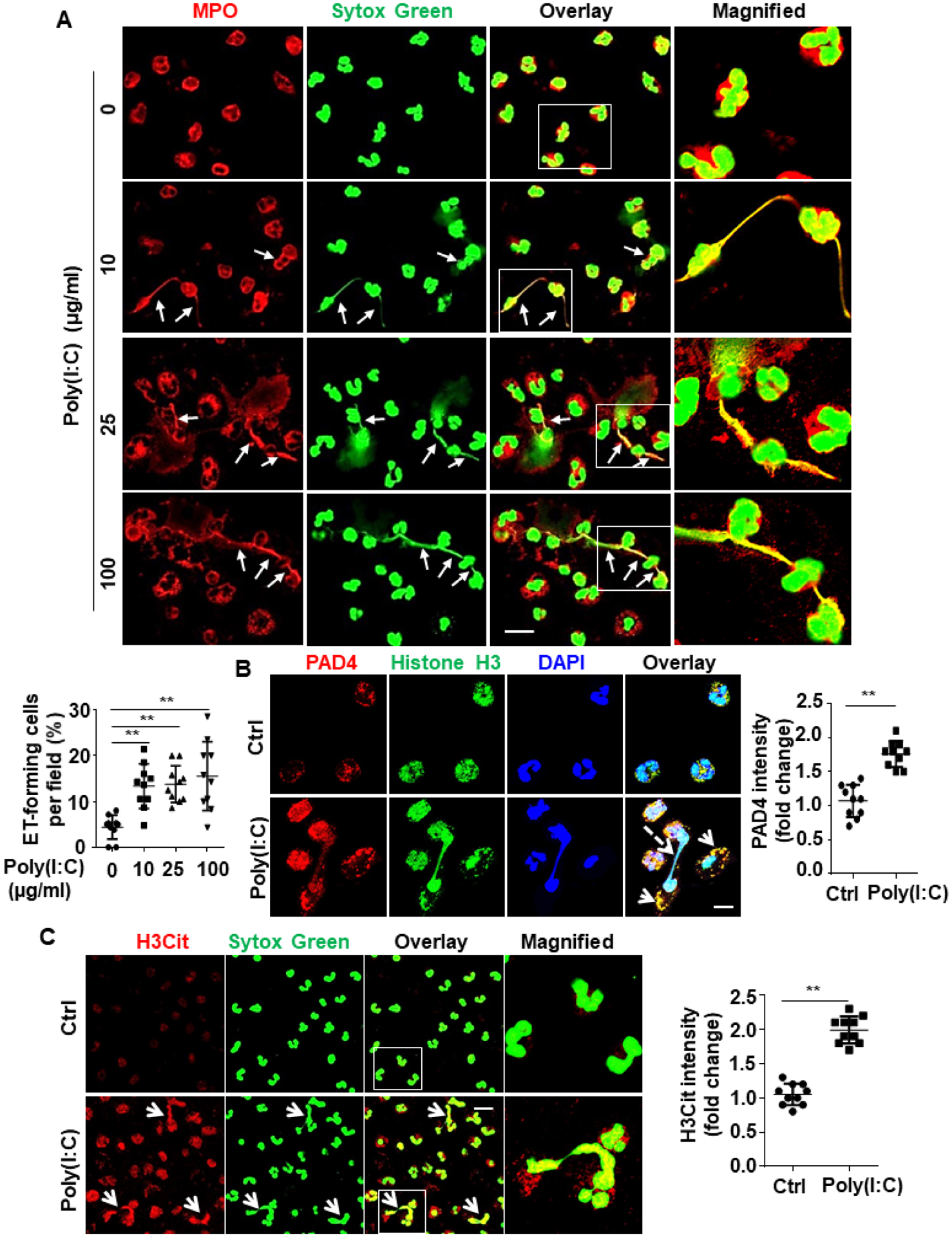
(A) Human blood neutrophils were isolated, cultured and stimulated with poly(I:C) for 4h at designated concentrations (10, 25, 100 μg/mL). Cells were then fixed, permeabilized and Myeloperoxidase (MPO), a neutrophil marker, was stained with rabbit anti-MPO antibody and Alexa Fluor 555-conjugated goat anti-rabbit IgG. DNA was stained with Sytox Green. Immunofluorescence images were taken by confocal microscopy. Arrows indicate cells with extracellular trap formation. The inset boxes from each group are magnified. Dot plot shows quantification of NET-forming cells. (B) Human blood neutrophils were isolated, cultured and stimulated with poly(I:C) (10 μg/mL for 4h). Cells were then fixed, permeabilized and histone H3 was stained with mouse anti-histone H3 antibody and Alexa Fluor 488-conjugated goat anti-mouse IgG. Peptidylarginine deiminase 4 (PAD4) was stained with rabbit anti-PAD4 antibody and Alexa Fluor 555-conjugated goat anti-rabbit IgG. Immunofluorescence images were taken by confocal microscopy. Arrows indicate the colocalization of PAD4 with histone H3. Dashed arrows indicate cells with NET formation. Dot plot shows quantitation of the relative expression levels of PAD4. (C) Human blood neutrophils were isolated, cultured and stimulated with poly(I:C) (10 μg/mL for 4h). Cells were then fixed, permeabilized and citrullinated histone H3 (H3Cit) was stained with rabbit anti-H3Cit antibody and Alexa Fluor 555-conjugated goat anti-rabbit IgG. DNA was stained with Sytox Green. Immunofluorescence images were taken by confocal microscopy. Arrows indicate cells with NET formation. The magnified insets correspond to the cells marked with white boxes. Dot plot shows quantitation of the relative expression levels of H3Cit. All experiments were repeated at least three times. **p < 0.01. Ctrl, control. Scale bar: 10 μm (B), 20 μm (A and C).
