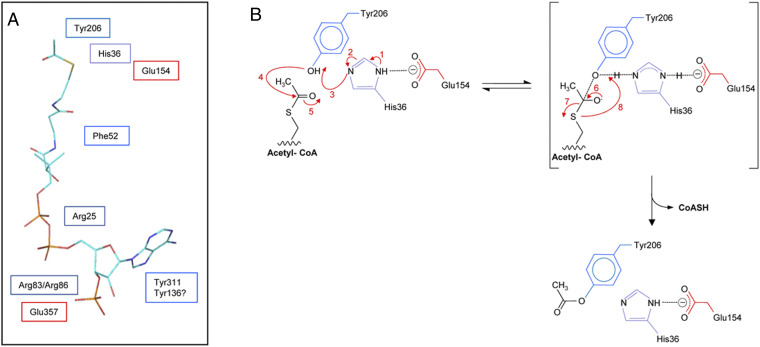
Fig. 5.
Proposed binding site of acetyl-CoA and mechanism of action of SaOatAN. (A) Proposed positioning of residues identified as essential for SaOatAN activity relative to acetyl-CoA. The structural conformation of acetyl-CoA presented was adopted from its complex with galactoside acetyltransferase as determined by X-ray crystallography (57) (Protein Data Bank Acc. No. 1KRR). (B) Postulated mechanism of SaOatAN. Aided by its interaction with Glu154 (reaction 1, 2; red labeling), His36 acts as a base to abstract the proton from the hydroxyl group of Tyr206 (reaction 3), rendering it nucleophilic. An attack on the carbonyl carbon of the thioester of acetyl-CoA by the nucleophilic Oη of Tyr206 (reaction 4) leads to a putative tetrahedral oxyanion intermediate (reaction 5), which collapses (reaction 6) to release CoASH (reaction 7, 8) and the formation of the O-acetyl-Tyr206 product.