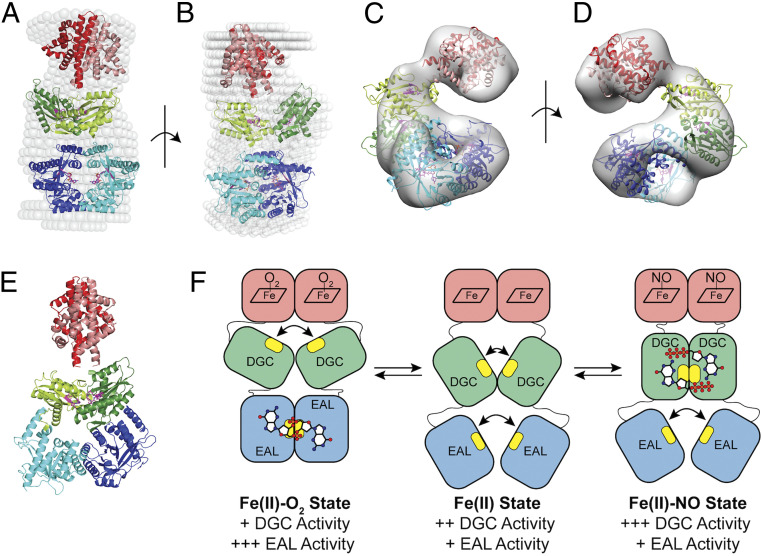
Fig. 6.
SAXS and negative stain models of DcpG Fe(II)-O2. (A and B) Two perpendicular views of an overlay of the SAXS solution envelope (white spheres) on the DcpG EAL active dimer model. Monomer 1 is shown in darker colors, while monomer 2 is depicted in lighter shades. The EAL domains (blue) form a ring stabilizing the active site residues inside. The GGDEF domains (green) are in an inactive conformation with their active sites ∼17 Å apart; globin domains are shown in red. (C and D) Two views (180° rotation) of DcpG negative stain EM density and model (ribbon colors are the same as in the SAXS model; σ level = 0.600). (E) A model of DcpG that allow dimerization and activity of the GGDEF domains. The active sites, shown in magenta, are ∼7 Å apart, as seen in other diguanylate cyclase active dimer structures. (PDB: 4URG, 6EIB, and 3I5B) (83–85). The EAL dimer is in a nonproductive form also seen in an earlier inactive dimer structure (PDB: 4RNI) (81). (F) A cartoon model of the DcpG conformational states. Active sites are highlighted in yellow, with GTP and c-di-GMP depicted as ball/stick models. Relative in vitro activity of the enzymatic domains is shown below.