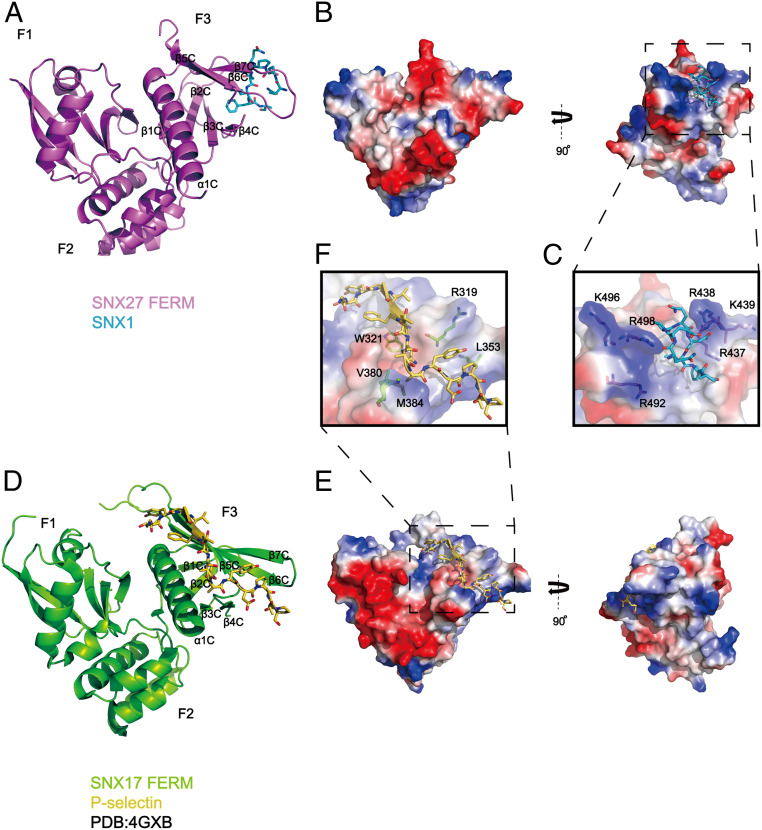
Fig. 2.
Crystal structure of SNX27-FERM-SNX1 and its comparison with the SNX17-FERM-P-selectin structure. (A) Ribbon diagram of SNX27 FERM domain (violet) in complex with the SNX1 peptide, which is represented as cyan sticks. The three submodules of SNX27 FERM, F1, F2, and F3, are marked, and the secondary structure of F3 is labeled. (B) The overall structure is shown in two perpendicular views depicted by SNX27 surface colored for electrostatic potential, with SNX183-90 peptide colored in cyan. Red and dark blue colors indicate negatively or positively charged surfaces of SNX27 FERM, separately. White colors highlight the hydrophobic region on SNX27 FERM surface. (C) A close-up inset image of the interface in the dashed line box shown in B. SNX27 is represented as a partially transparent surface with specific positively charged side chains around the hydrophobic binding pocket in accommodation for SNX1. (D) SNX17 in complex with cargo P-selectin (Protein Data Bank: 4GXB) is depicted with analogous representations to those shown for SNX27 in A. (E) SNX17 in complex with cargo P-selectin is represented similarly to SNX27 shown in B. (F) A zoomed-in picture of the interface between SNX17 and P-selectin in the dashed line box shown in E. A partially transparent electrostatic surface potential map of SNX17 is presented. Critical residues involved in interacting with P-selectin are shown in stick mode.