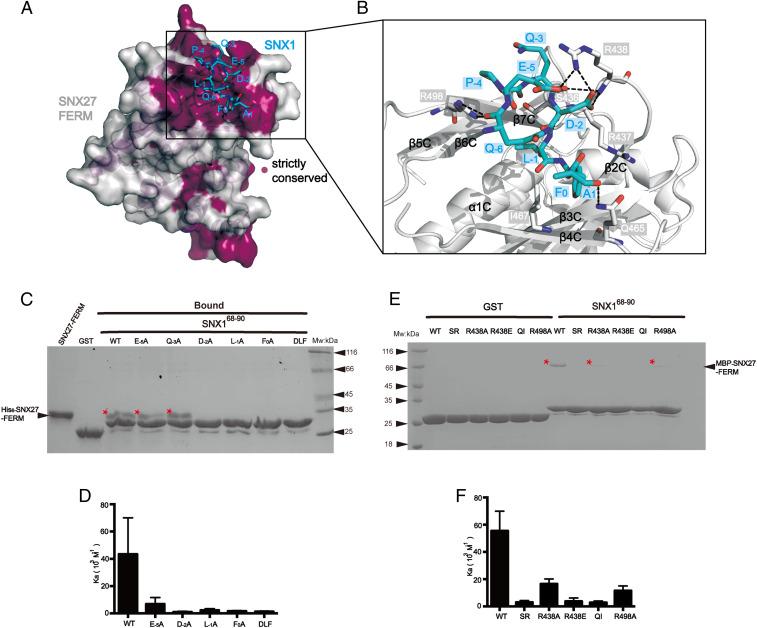
Fig. 4.
Structural mechanism of recognition of the DLF motif from SNX1 by SNX27 FERM. (A) Conservation analysis of the FERM domain of SNX27 using the ConSurf Server (https://consurf.tau.ac.il) shows that the SNX1-binding groove among the F3 submodule is strictly conserved. The surface of SNX27-FERM is colored as light gray (nonconserved) and purple (strictly conserved). SNX1 peptide is shown in cyan sticks. (B) Close-up view showing the detailed binding between the SNX1 DLF motif (cyan) and SNX27-FERM (partially transparent surface; blue and red represent positive and negative charge, respectively). Critical residues on SNX27-FERM important for SNX1 recognition are highlighted in stick mode; black dashed lines indicate the observed hydrogen bonds and salt bridges in the structure. (C) GST, GST-SNX168-90 WT, and mutants (E−5A, Q−3A, D−2A, L−1A, F0A, and DLF) pull down His6-SNX27-FEEM. Shown is a Coomassie Blue–stained SDS/PAGE gel of bound samples of GST in right and GST-SNX1 in left. * indicates the retained His6-SNX27-FERM. (D) Binding affinity between MBP-SNX27-FERM protein and WT or corresponding mutants of SNX168-90 peptides in C determined by ITC. Association constant (Ka) is shown together with errors from data fitting. (E) GST and GST-SNX168-90 pull-down of purified MBP-SNX27-FERM WT, SR, R438A, R438E, QI, and R498A, respectively. Shown is a Coomassie Blue–stained SDS/PAGE gel of bound samples of GST on the right and GST-SNX1 on the left. * indicates the retained MBP-SNX27-FERM. (F) Binding affinity between MBP-SNX27-FERM WT or mutants in E and SNX168-90 WT determined by ITC. Association constant (Ka) is shown together with errors from data fitting.