Figure 1: Participant disposition and study treatment exposure.
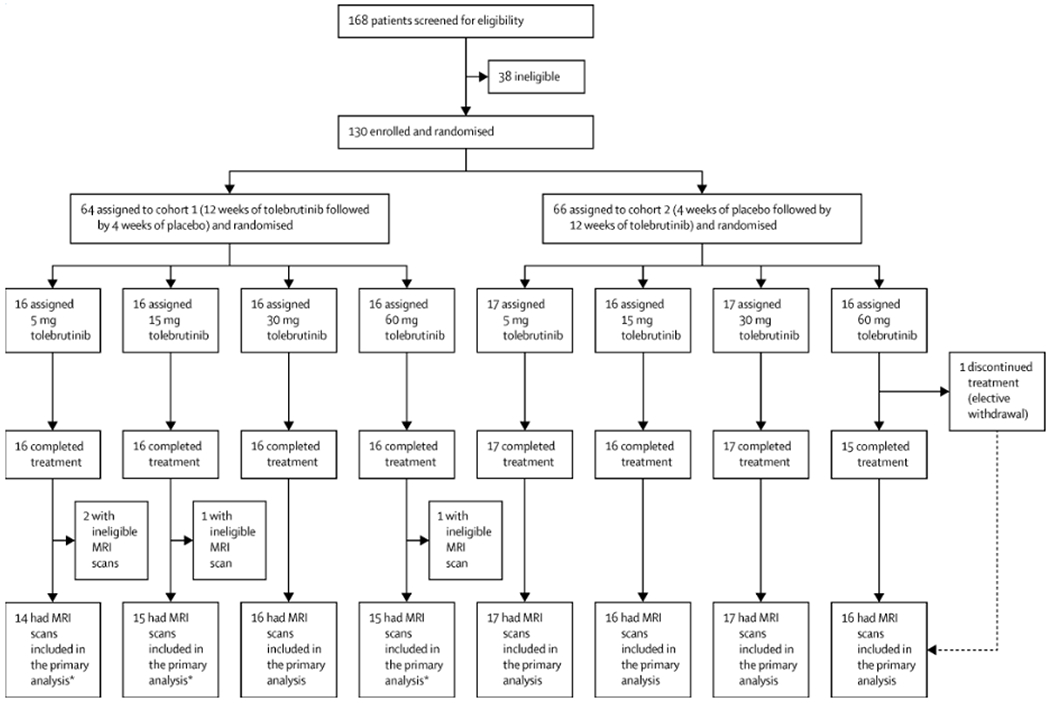
Participants were randomised in two steps: first (1:1) into two cohorts and then (1:1:1:1) into four dosage groups within each cohort. equally into one of eight treatment arms that were divided between two cohorts. Cohort 1 received tolebrutinib for 12 weeks beginning at baseline, followed by 4 weeks of placebo run-out in order to blind the treatment assignment and to provide additional safety data. Cohort 2 received placebo for 4 weeks as a run-in, followed by tolebrutinib for 12 weeks. Of the 130 participants randomised, 129 completed the treatment, and the participant with early treatment discontinuation still completed the study. For the primary analysis, an MRI assessment was excluded from analysis if the participant had received systemic corticosteroids within the 30 days prior to the MRI assessment date. Thus, MRI assessments from 126 of 130 participants were included in the primary analysis (Cohort 1: 5 mg, n=14; 15 mg, n=15; 30 mg, n=16; 60 mg, n=15. Cohort 2: 5 mg, n=17; 15 mg, n=16; 30 mg, n=17; 60 mg, n=16). R=randomisation.
