Figure 2: Primary outcome: Number of new Gd-enhancing lesions on an MRI scan after 12 weeks of tolebrutinib treatment.
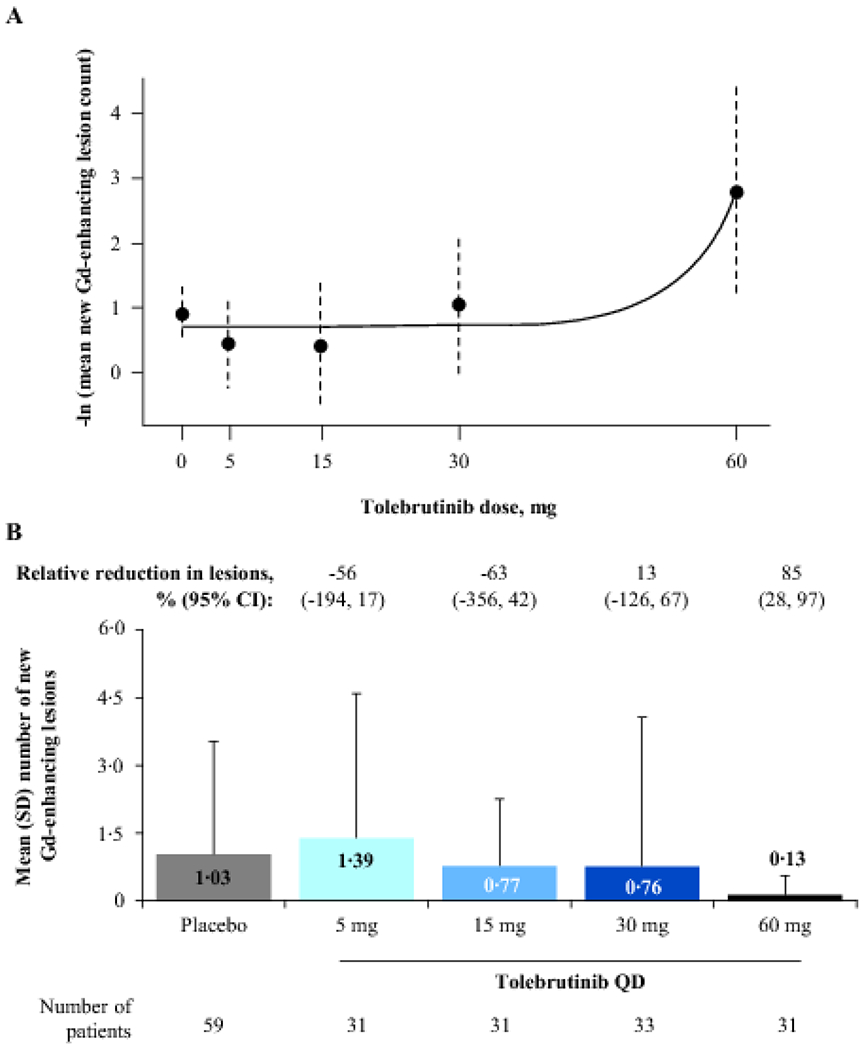
Panel A shows the estimated dose-response curve after applying the two-step MCP-Mod methodology; error bars represent 95% CIs. The exponential model was selected as the best fit based on the Akaike information criterion and was used to reject the null hypothesis (p=0·03). Panel B shows the mean (error bars: SD) number of new Gd-enhancing lesions per scan for pooled participants from Cohorts 1 and 2 at the end of 12 weeks of tolebrutinib treatment compared with a scan 4 weeks prior. In Cohort 2, the formation rate of new Gd-enhancing lesions over the 4-week placebo run-in period was assumed to be constant over the 16-week trial duration. Values are relative reduction in lesion count versus placebo, adjusted for presence or absence of baseline Gd-enhancing lesions, using a negative binomial model. CI=confidence interval. Gd=gadolinium. MCP-Mod=multiple comparison procedure-modelling. MRI=magnetic resonance imaging. QD=once daily. SD=standard deviation.
