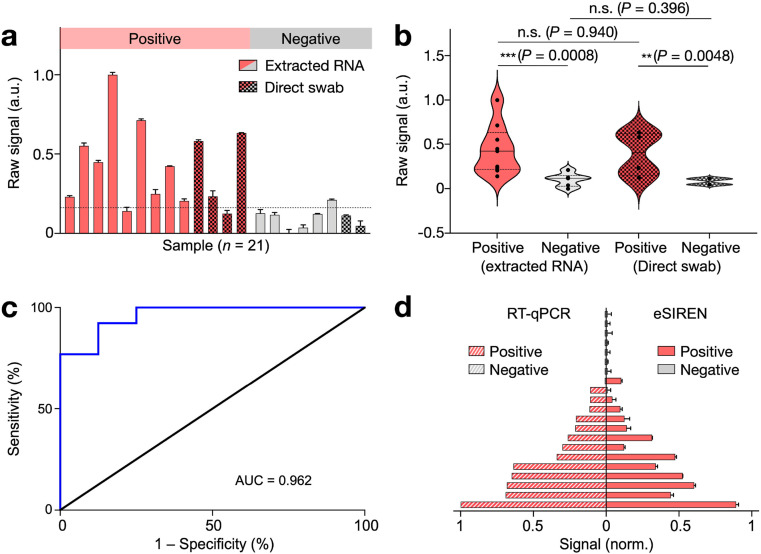
Fig. 5.
Clinical validation of eSIREN for COVID-19 diagnosis.
(a) eSIREN measurements were performed on extracted RNA samples (n = 15) as well as heat-inactivated swab samples (n = 6). Out of the 21 clinical samples, 13 samples were determined as positive and 8 as negative for COVID-19 infection using gold-standard RT-qPCR assay. (b) Statistical analysis of the eSIREN results. Across both extracted RNA and direct swab samples, the positive samples showed an elevated signal compared to the negative samples. (c) Receiver operator characteristic (ROC) curve of the eSIREN results. The platform demonstrated a high accuracy for SARS-CoV-2 detection (area under curve, AUC = 0.962). (d) eSIREN results for clinical classification. By applying an optimal assay threshold based on the Youden's index (the dotted line as indicated in a), the eSIREN platform showed sensitivity = 92.3%, specificity = 87.5% and overall accuracy = 90.5% across all tested clinical samples. The comparisons were performed against clinical RT-qPCR results of matched samples. All measurements were performed in triplicate. In a, c, the data are presented as mean ± s.d; in b, the data are presented as mean. (n.s., not significant, Student's t-test).. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)