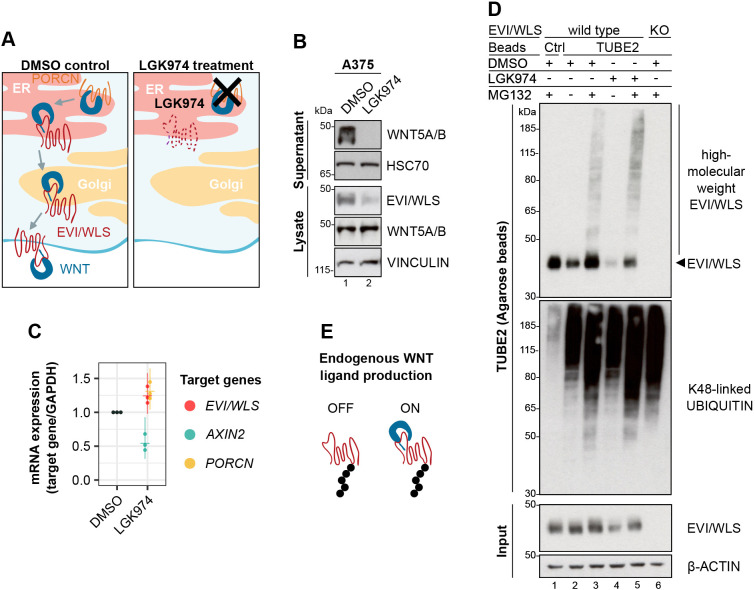
Fig. 5.
EVI/WLS is ubiquitylated in cells with endogenous WNT ligands. (A) Schematic illustration of the mode of action of LGK974. LGK974 prevents WNT ligands from being lipid-modified in the ER by inhibiting the acyl-transferase PORCN. Unlipidated WNTs cannot associate with EVI/WLS and are not secreted from the WNT producing cell. (B,C) LGK974 treatment reduced intracellular EVI/WLS levels and abolished the secretion of WNT5A and/or WNT5B (WNT5A/B) without reducing EVI/WLS gene expression. A375 melanoma cells were treated with LGK974 (10 µM) or DMSO for 96 h with daily medium changes. (B) Secreted proteins were precipitated from the supernatant using Blue Sepharose, and relative WNT5A/B levels were compared to those in cell lysates. Vinculin and HSC70 served as loading controls. (C) Target gene expression was normalised to DMSO treatment and GAPDH served as reference gene. Individual data points from three independent experiments are shown with mean and 95% confidence intervals. (D) Ubiquitylated EVI/WLS accumulated after inhibition of the proteasome, independent of LGK974 treatment. Wild-type and EVI/WLS knockout (KO) A375 melanoma cells were treated with LGK974 (10 µM) or DMSO for 96 h with daily medium changes. Samples were treated with the proteasome inhibitor MG132 (1 µM), as indicated, 24 h before harvesting. Then, total cell lysates were sampled for input control (∼6.5 µg of total lysate) or used for TUBE2 (agarose) pulldown to precipitate polyubiquitylated proteins. Ubiquitin non-binding control (Ctrl) agarose beads showed a level of unspecific binding, and EVI/WLSKO cells confirmed specificity for EVI/WLS. β-actin served as loading control. (E) EVI/WLS is ubiquitylated in the presence (ON) and absence (OFF) of endogenous WNT ligands. Western blots in B and D are representative of three independent experiments.