Abstract
We compared the prognosis of inpatients with a known diagnosis of Alzheimer's or Parkinson's disease who have COVID-19 infection with other hospitalized patients with COVID-19. Our cohort study started in October 2020 and ended in May 2021 and included inpatients with COVID-19 infection who were admitted to hospitals. From a total of 67,871 patients with a confirmed diagnosis of COVID-19, a sample of 3732 individuals were selected of which 363 had Alzheimer's, and 259 had Parkinson's disease. All patients had both positive RT-PCR test and positive chest CT for COVID-19. The outcome was dead within 28 days of admission and the predictors were a large number of demographic and clinical features, and comorbidities recorded at patients’ bedside. Mortality were 37.5%, 35.1%, and 29.5% in patients with Alzheimer's disease, Parkinson's disease; and in other patients, respectively. The hazard ratio for Alzheimer's disease was 1.27 (95% CI, 1.06–1.53, p = 0.010) and for Parkinson's disease was 1.17 (95% CI, 0.94–1.46, p = 0.171). Age was a predictor of mortality, hazard ratio = 1.04 (95% CI, 1.03–1.05, p < 0.001). Patients with Alzheimer's disease and COVID-19 infection were older and more likely to have a loss of consciousness on admission (both p ≤ 0.001). We concluded that inpatients with Alzheimer's disease have an increased risk for 28-day mortality from COVID-19 and healthcare settings should be ready to provide critical care for them such as early intubation and immediate O2 therapy. However, Parkinson's disease does not significantly predict higher mortality of COVID-19.
Keywords: Alzheimer, Parkinson, COVID-19, Neurodegenerative, Mortality, Survival
1. Introduction
Reducing mortality of COVID-19 requires the development of risk-based strategies [1]. Risk stratification allows to identify patients with a higher likelihood for mortality and to prioritize the allocation of health resources [2]. Overall, inpatient mortality from COVID-19 is 15–20%, and 40% of hospitalized patients require intensive care [3]. However, reports from different populations show variability in mortality rates of COVID-19 [1]. Hospital mortality is estimated to be less than 5% for individuals younger than 40 years, 35% for patients aged 70 to 79 years, and more than 60% for people 80 to 89 years of age [4]. Individuals undergoing kidney transplant or dialysis; and patients with cancer, diabetes, neurologic or cardiovascular diseases are suggested to be at increased risk for mortality [5], [6], [7], [8], [9].
Research studies suggested that SARS-CoV-2 can invade the central nervous system and initiate neurological manifestations [10], [11]. Dementia has been reported as common comorbidity in patients with COVID-19 [12], [13]. Also, Alzheimer's disease (AD) is suspected of increasing the mortality rate of COVID-19 among inpatients and people living in care homes [14], [15], [16]. The restrictions imposed due to the COVID-19 pandemic are capable of worsening motor and non-motor symptoms in Parkinson's disease (PD) [17]. Studies suggested that case fatality is higher in patients having COVID-19 and Parkinson's disease [18]. However, some researchers suggested that PD does not increase mortality from COVID-19 [19].
Overall, the study design, populations, sample size, and the diagnostic criteria of COVID-19 varied between studies on the mortality risk of COVID-19 in patients with neurodegenerative diseases (NDD) [20]. These inconsistencies lead to inconclusive mortality data and necessitate further epidemiological studies [21], [22]. Particularly, a longitudinal follow-up of patients is required to reveal the impact of NDD on mortality of COVID-19 [23].
Early at the onset of COVID-19, findings on chest computerized tomography (CT) include ground-glass opacity with bilateral peripheral involvement in multiple lobes and consolidation [24]. The disease is commonly diagnosed using a reverse transcription-polymerase chain reaction (RT-PCR) test with 20% to 67% false-negative based on time since exposure [25]. In patients with clinical suspicion of COVID-19, the negative test result should be interpreted together with other clinical and paraclinical evidence [25].
The aim of conducting this longitudinal study was to compare the prognosis of inpatients with a known diagnosis of AD or PD who have COVID-19 infection with other hospitalized people with COVID-19. We researched the risk of mortality within 28 days of admission using a large number of clinical indicators. The diagnosis of COVID-19 infection was confirmed by both RT-PCR and chest CT for all patients. We hypothesized that the three groups would differ in mortality risk of COVID-19.
2. Patients and methods
2.1. Design and settings
We assessed the risk for mortality of COVID-19 among patients with and without NDD. Our multicenter cohort study of hospitalized patients started in October 25, 2020 and ended in May 27, 2021. The target population was people with COVID-19 infection who were admitted to university healthcare centers. This research was conducted by the Critical Care Quality Improvement Research Center, affiliated with Shahid Beheshti University of Medical Sciences. We gathered data with the help of 63 medical universities in their 816 affiliated healthcare centers. Ethics approval was obtained from the Institutional Review Boards of Shahid Beheshti University of Medical Sciences with the ethics code of IR.SBMU.RETECH.REC.1399.499. At the admission time, written consent was taken from all participants or their companions. No personally identifiable information including the patient's name and social security number was entered into the analytical data.
2.2. Eligibility
We included all people who were hospitalized with a confirmed diagnosis of COVID-19 infection. All patients were monitored for progressive signs of respiratory failure and shock. Acute respiratory distress syndrome was diagnosed based on the Berlin definition [26]. We used pharmacological prophylaxis to prevent thromboembolism when not contraindicated. The complications associated with critical care, such as ventilator-associated pneumonia and catheter-related bloodstream infection, were prevented according to the standard practice [27], [28]. The registration process was online using a single electronic form. Patients were considered as having the new coronavirus infection if they had a positive RT-PCR test plus a positive chest CT finding for COVID-19. The RT-PCR result was reported based on testing collected specimens from the upper respiratory tract. Radiologists or pulmonologists read CT images using the guidelines reported in the literature [29]. Both RT-PCR test results and CT findings were recorded as binary values. People with AD or PD were identified with their past medical history and records. At each healthcare center a general practitioner was responsible for data collection. At the end of the process of patient pooling, we had three groups of COVID-19 (COV), COV + AD, and COV + PD.
2.3. Outcome and predictors
The outcome was dead within 28 days of admission. Because there is still no definitive cure for COVID-19 infection we considered that the predictive effect of the interventions is small compared with the other predictors. Most of the data were collected early in admission and later in reviewing patients’ medical records. All predictors were clinical features and were recorded easily at patients’ bedside to achieve high practicality. Collected data were saved as a Microsoft Excel® spreadsheet. For resolving the imbalance in NDD we carried out random sampling of the COV group. Predictors with a less than 5% frequency for at least one of its levels were considered as highly imbalanced and were excluded from further analysis. There were no missing data for the outcome variable and duration of hospital stay. We did not recognize any possibility for lacking randomness for missing data in predictors and imputed them.
2.4. Statistical analyses and modeling
Results are presented as mean (SD) for continuous variables and as absolute numbers (percentage) for categorical data. The means of the continuous variables were compared using t-test or Analysis of Variance (ANOVA) tests where appropriate. Either a χ2 or Fisher exact test was used for testing differences among the groups for categorical variables. For statistical analyses, p-values less than 0.05 were considered significant. Median hospital stay (day) was compared among three groups with the Kruskal-Wallis test, and between two groups with the Wilcoxon rank sum test. The survival curves for the three groups of patients were constructed with the Kaplan-Meier method and were compared with the log-rank test. The Cox proportional-hazards model was used to investigate the association between survival time and predictors. We used R software version 4.0.2 for data analysis and visualization. R is a free software environment, well known for its statistical and machine learning libraries and graphics. We used a variety of R packages for the analysis. All the packages were downloaded from the Comprehensive R Archive Network (https://cran.r-project.org/), the official R package repository, or the GitHub (https://github.com/) website.
3. Results
3.1. Sample
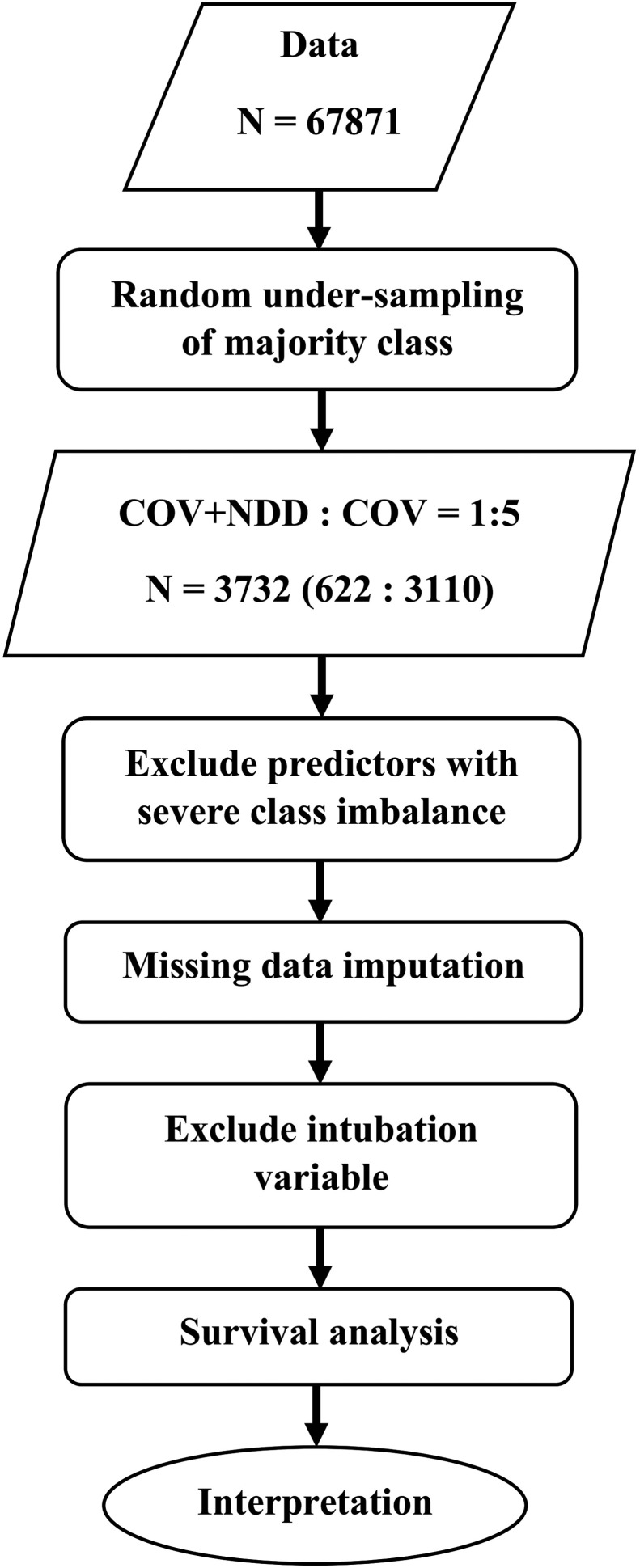
We had 67,871 patients with a confirmed diagnosis of COVID-19 of which 622 (0.9%) had NDD. Of the patients with NDD, 363 (58.4%) had AD, and 259 (41.6%) had PD. Because of severe imbalance in the number of patients with NDD, we randomly under-sampled the COV group to provide 1:5 ratios of COV + NDD: COV patients in the final sample. Each COV patient was randomly selected as an individually age- and sex-matched to NDD patients. At the end of the process, the analytic sample included 3732 patients (3110 COV, 363 COV + AD, and 259 COV + PD) and 320 (0.3%) missing data. The missing data included 247 (6.6%) for the respiratory rate, 64 (1.7%) for O2 therapy, 3 (0.08%) for PaO2, 3 (0.08%) for the temperature at admission, and 3 (0.08%) for the day of beginning the symptoms before admission. The missing data were imputed using predictive mean matching. We excluded highly imbalanced predictors from the analysis including seizure, paresis or plegia, dermatologic and hematologic problems, liver diseases, HIV/AIDS, cancer, chemotherapy or immune deficiency, loss of smell or taste, abdominal or chest pain, vomiting, diarrhea, vertigo, urological disease or dialysis, smoking or drug abuse, and asthma. Fig. 1 shows the flow of data in this study.
Fig. 1.
Data flow chart of the study. COV: patient with a confirmed diagnosis of COVID-19 infection; NDD: neurodegenerative diseases.
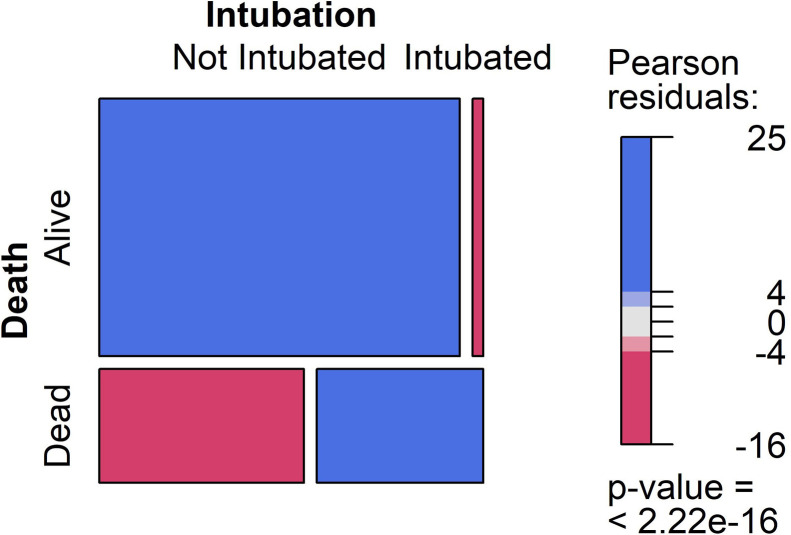
Based on the remaining features, Table 1 shows the characteristics of the two groups of patients. Mortality rates were 29.5%, 37.5%, and 35.1% in COV, COV + AD, and COV + PD, respectively. The percentages of intubation, O2 therapy on admission, loss of consciousness, respiratory distress, systolic hypertension, diabetes, cardiovascular disease, and AD were significantly higher and the percentages of myalgia and headache were significantly lower in dead patients. Also, there was a significant difference in respiratory rate categories between alive and dead patients. In addition, dead people were older and presented with a lower PaO2 on admission. It seems that the symptoms progressed more quickly in the dead group before admission. Also, comparisons of the three groups indicated that patients with COV + AD were the oldest and mostly women. They comprised the largest percentage of O2 therapy and loss of consciousness on admission. The COV group showed the largest percentage of myalgia, cough, nausea, and headache. Of the measured predictors, intubation on admission was highly significant in predicting the poor outcome of hospitalization (Table 1). Fig. 2 shows the mosaic plot for comparing not intubated with intubated patients in the proportion of mortality. The odds ratio of intubation for in-hospital mortality was 27.2 with the 95%CI of 21.1 to 35.2. We excluded intubation from further analyses as it highly influenced the process of modeling.
Table 1.
Patients’ characteristics in alive and dead groups.
| Feature | Group |
Group |
|||||
|---|---|---|---|---|---|---|---|
| Alive (n = 2586) | Dead (n = 1146) | p | COV (n = 3110) | COV + AD (n = 363) | COV + PD (n =259) | p | |
| Intubation (%) | 75 (2.9) | 514 (44.9) | < 0.001* | 476 (15.3) | 67 (18.5) | 46 (17.8) | 0.197 |
| Mean (SD) age (year) | 76.1 (10.1) | 79.7 (8.3) | < 0.001* | 77.2 (9.7) | 80.2 (7.9) | 73 (10.5) | 0.001* |
| Woman (%) | 978 (37.8) | 420 (36.6) | 0.519 | 1165 (37.5) | 153 (42.1) | 80 (30.9) | 0.017* |
| Mean (SD) PaO2 (mmHg) | 88.4 (7.9) | 81.9 (13.1) | < 0.001* | 86.5 (10.2) | 85.5 (10.7) | 86.3 (9.7) | 0.271 |
| O2 therapy early at admission (%) | 1293 (50.0) | 687 (59.9) | < 0.001* | 1635 (52.6) | 215 (59.2) | 130 (50.2) | 0.035* |
| Respiratory Rate (%) | |||||||
| < 14 | 4 (0.2) | 13 (1.1) | < 0.001* | 14 (0.5) | 2 (0.6) | 1 (0.4) | 0.921 |
| 14–18 | 669 (25.9) | 246 (21.5) | 770 (24.8) | 83 (22.9) | 62 (23.9) | ||
| 18–22 | 1417 (54.8) | 560 (48.9) | 1656 (53.2) | 188 (51.8) | 133 (51.4) | ||
| 22–28 | 431 (16.7) | 263 (22.9) | 564 (18.1) | 76 (20.9) | 54 (20.8) | ||
| > 28 | 65 (2.5) | 64 (5.6) | 106 (3.4) | 14 (3.9) | 9 (3.5) | ||
| Mean (SD) Temperature (°C) | 37.2 (1.6) | 37.2 (0.7) | 0.785 | 37.2 (1.5) | 37.2 (0.7) | 37.3 (0.7) | 0.989 |
| Fever (%) | 837 (32.4) | 382 (33.3) | 0.587 | 1004 (32.3) | 121 (33.3) | 94 (36.3) | 0.4 |
| Loss of Consciousness (%) | 153 (5.9) | 210 (18.3) | < 0.001* | 238 (7.7) | 81 (22.3) | 44 (17) | < 0.001* |
| Respiratory Distress (%) | 1451 (56.1) | 813 (70.9) | < 0.001* | 1911 (61.4) | 202 (55.6) | 151 (58.3) | 0.073 |
| Myalgia (%) | 803 (31.1) | 305 (26.6) | 0.007* | 997 (32.1) | 59 (16.3) | 52 (20.1) | < 0.001* |
| Cough (%) | 1105 (42.7) | 486 (42.4) | 0.883 | 1383 (44.5) | 125 (34.4) | 83 (32.0) | < 0.001* |
| Systolic Hypertension (%) | 821 (31.7) | 410 (35.8) | 0.017* | 1019 (32.8) | 117 (32.2) | 95 (36.7) | 0.415 |
| Nausea (%) | 219 (8.5) | 75 (6.5) | 0.052† | 268 (8.6) | 13 (3.6) | 13 (5.0) | < 0.001* |
| Anorexia (%) | 397 (15.4) | 150 (13.1) | 0.08 | 459 (14.8) | 53 (14.6) | 35 (13.5) | 0.862 |
| Headache (%) | 218 (8.4) | 57 (5.0) | < 0.001* | 252 (8.1) | 10 (2.8) | 13 (5.0) | < 0.001* |
| Diabetes (%) | 529 (20.5) | 268 (23.4) | 0.049* | 672 (21.6) | 80 (22) | 45 (17.4) | 0.264 |
| Cardiovascular Disease (%) | 470 (18.2) | 274 (23.9) | < 0.001* | 640 (20.6) | 57 (15.7) | 47 (18.1) | 0.067† |
| Positive Contact History (%) | 1203 (46.5) | 537 (46.9) | 0.876 | 1452 (46.7) | 170 (46.8) | 118 (45.6) | 0.937 |
| Mean (SD) Start of symptoms before admission (days) | 5.6 (3.9) | 5.3 (4.1) | 0.042* | 5.5 (3.9) | 5.4 (4.4) | 5.7 (4.5) | 0.594 |
| COV (%) | 2191 (84.7) | 919 (80.2) | < 0.001* | ||||
| COV + AD (%) | 227 (8.8) | 136 (11.9) | 0.004* | ||||
| COV + PD (%) | 168 (6.5) | 91 (7.9) | 0.127 | ||||
Categorical variables were compared with χ2, and continuous variables were compared with t-test for independent samples between alive and dead groups, and with ANOVA test among COV, COV + AD, and COV + PD groups. SD: Standard Deviation; PaO2: Partial Pressure of Oxygen; COV: Patient with a confirmed diagnosis of COVID-19 infection; AD: Alzheimer's Disease; PD: Parkinson's Disease.
Significant at p < 0.05.
Approaching significance.
Fig. 2.
Mosaic plot for death vs. intubation. The column widths indicate the relative proportions of the corresponding values at each variable. The blue color means there are more observations in that cell than would have been expected under the null hypothesis of independence. The red color indicates that there are fewer observations than would have been expected.
3.2. Survival analysis
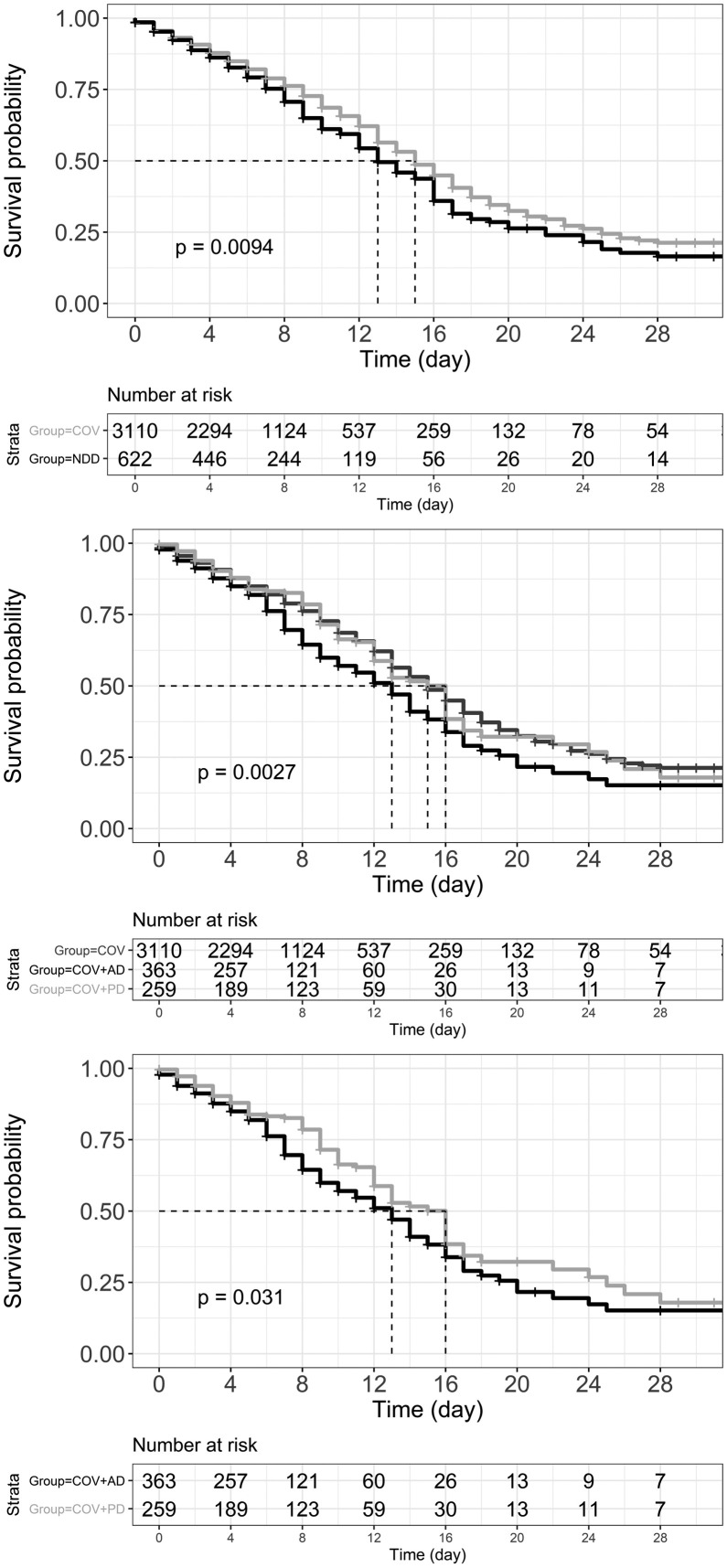
The median hospital stay was 6, 6, and 7 days for COV, COV + AD, and COV + PD groups (Kruskal-Wallis χ2(2) = 5.4429, p = 0.066). Pairwise Wilcoxon rank sum test showed that there is a significant difference between COV + AD and COV + PD in median hospital stay (W = 42,008, p = 0.023). Fig. 3 illustrates Kaplan-Meier curves for survival probability. Early at admission, the curves are parallel, however, they separate gradually with a steeper decline in the COV + AD group. The Cox proportional-hazards model incorporating fixed (non–time-dependent) covariates significantly predicted the risk for mortality; Wald χ2(8) = 122.8, p < 0.001 (Table 2 ). Hazard ratios showed that AD is a significant predictor of mortality in patients with COVID-19 infection.
Fig. 3.
Kaplan-Meier curve of survival probability. The dashed lines represent median survival and p values show if there is a difference between the survival curves using rank tests. Top: COV (light gray) and NDD (black) groups. Middle: COV (dark gray), COV + AD (black), and COV + PD (light gray). Bottom: COV + AD (black) and COV + PD (light gray). COV: Patient with a confirmed diagnosis of COVID-19 infection; AD: Alzheimer's Disease; PD: Parkinson's Disease.
Table 2.
Cox proportional-hazards regression for the survival data.
| 95% CI |
||||
|---|---|---|---|---|
| Feature | Hazard | Lower Limit | Upper Limit | p |
| PD | 1.17 | 0.94 | 1.46 | 0.171 |
| AD | 1.27 | 1.06 | 1.53 | 0.010* |
| Age (year) | 1.04 | 1.03 | 1.05 | < 0.001* |
| Sex (male) | 1.07 | 0.95 | 1.21 | 0.254 |
| Diabetes | 1.15 | 0.99 | 1.33 | 0.057† |
| Cardiovascular Disease | 1.12 | 0.98 | 1.29 | 0.105 |
| Contact History | 0.98 | 0.87 | 1.10 | 0.679 |
| Start of symptoms before admission (days) | 0.97 | 0.96 | 0.99 | 0.005* |
AD: Alzheimer's Disease; PD: Parkinson's Disease.
Significant at p < 0.05.
Approaching significance.
4. Discussion
This study was conducted to investigate the effects of AD and PD on the prognosis of hospitalized patients with COVID-19 infection. We included patients diagnosed as having COVID-19 infection using both RT-PCR and chest CT findings and evaluated their risk of mortality within 28 days of admission. A large number of predictors were recorded including demographic features, clinical manifestations, and comorbidities.
Our data showed that patients who died were older and presented to the healthcare centers with more critical symptoms such as loss of consciousness, respiratory distress, systolic hypertension, and lower PaO2. They had diabetes or cardiovascular disease more frequently and needed early intubation and O2 therapy more than patients with good prognoses. Also, dead people showed relatively faster progress of symptoms before admission. Meanwhile, patients who remained alive during the first 28 days of hospitalization experienced myalgia and headache more frequently. We found that AD significantly affects the risk for mortality. Patients with COV + AD were older than the other groups and were mostly women. They also were frequently presented to hospitals with more serious manifestations such as loss of consciousness and needed more serious care such as early intubation and O2 therapy compared with COV and COV + PD groups. In contrast, patients without NDD experienced myalgia, cough, nausea, and headache more commonly compared with the NDD group.
Matias-Guiu et al. carried out a case series study to evaluate the frequency and mortality of COVID-19 in patients with AD and frontotemporal dementia. They included 204 individuals; 147 patients with AD and 57 with frontotemporal dementia. Overall, 22 (15.0%) of patients had COVID-19 and AD. They reported that in patients with COVID-19, 12 out of 22 (54.5%) patients with AD died and concluded that AD is associated with a higher risk for mortality than frontotemporal dementia. Also, they suggested that increasing age might be associated with a higher mortality rate while their logistic model did not assign a significant role to age [14]. Our study was longitudinal and quite larger; we found significant and independent effects for age and AD on mortality of COVID-19. Similarly, our AD group was older and mostly female. Of the 363 patients with COV + AD in our study, 136 (37.5% of AD patients) died which is smaller than the mortality rate of patients with AD in Matias-Guiu's study. Inclusion of patients with AD without stratification of severity and residency (home and care home) might have diluted our percentage.
Similar pathological changes in AD and COVID-19 might increase the risk for mortality in a synergistic manner. Commonly, AD affects patients more than 65 years of age with a prevalence that doubles every 5 years [30]. Also, aging is a known risk factor for mortality of COVID-19 [4]. In addition, the excessive expression of viral receptor angiotensin-converting enzyme 2 and pro-inflammatory molecules, the comorbidities of AD complications such as diabetes, lifestyle alterations in AD, and behavioral changes imposed by COVID-19 have been suggested to increase the risk for mortality of COVID-19 in patients with AD [11]. Inflammatory mediators have been suggested to induce CNS manifestations and immunological processes in the peripheral nervous system of patients with COVID-19 infection. Interleukin 6 and 1, cytoskeleton-associated protein 4, and galectin 9 are thought as the common links between COVID-19 and AD manifestations [31].
Scherbaum et al. carried out a cross-sectional nationwide assessment of hospitalized patients with PD in Germany and evaluated the impact of the COVID-19 pandemic. They reported that patients with PD aged 65 years or older were at higher risk of COVID-19 and that advanced age and male sex were more frequent in COVID-19. The COVID-19 inpatient mortality rate was higher in PD than in non-PD patients (35.4% vs. 20.7%, p < 0.001), particularly in patients aged 75–79 years. They concluded that PD inpatients are more frequently affected by COVID-19 and had increased COVID-19-associated mortality compared with non-PD patients. Meanwhile, they warranted that more studies are needed to assess the significance of associated comorbidities for COVID-19 risk and mortality in PD [32]. In our study, the mortality of the COV + PD group was 35.1% — similar to the Scherbaum study — and the mortality of COV was 29.5% — higher than the Scherbaum study. The mean (SD) age was 73 (10.5) years in our COV + PD group. They focused on age ranges, while we investigated the effects of a large number of predictors (including age). They conducted a cross-sectional study, but we carried out longitudinal research. They estimated a higher mortality for PD compared with the non-PD group. Our Kaplan-Meier curves showed that the COV + PD group had slightly less survival probability than the COV group. However, adjustments showed that in the presence of other predictors the difference was not significant. Considering the results of the two studies the discrepancy might be related to the higher mortality in our COV group rather than less mortality in our COV + PD group.
Nevertheless, another study performed by Vignatelli et al. indicated that PD per se is not a risk factor for COVID-19 hospitalization. They included patients with the clinical diagnosis of PD (n = 696) or Parkinsonism (n = 184) and people anonymously matched (ratio 1:10) for sex, age, district, and Charlson Index (n = 8590) in a historical cohort. The adjusted hazard ratio was 0.8 (95% CI, 0.3–2.3, p = 0.74) in PD. They also reported that the 30-day fatality rate was 35.1%, without difference among the 3 groups [33]. Our results are compatible with the Vignatelli study concerning mortality (35.1% for 28-day mortality in our study) and the PD hazard ratio of 1.17 (95% CI, 0.94–1.46, p = 0.171). In another study on health records of 13,338 UK individuals tested for COVID-19, Yu et al. showed that AD predicts the highest risk for mortality among elderly individuals; however, PD patients did not have a higher risk for mortality from COVID-19 [19]. It has been suggested that CoV-2 binds to the ACE2 receptors of the dopaminergic neurons of the striatum, which are degenerated by PD-related neuropathology [34]. This reduces neuroinvasion in those patients. Also, some PD medications have been thought to play therapeutic roles in COVID-19 [19].
We selected strict inclusion criteria (positive RT-PCR test result plus CT findings). This allowed us to be more confident regarding the diagnosis of COVID-19 infection. We assessed a large number of predictors including clinical manifestations and comorbidities. Our analyses were straightforward and we incorporated features that are quickly obtained by interview or physical examination upon admission of the patient. Meanwhile, we had some limitations in our study. We did not include lab tests in our predictor list. Recording binary variables prevented errors in data entry and led to a bit of ease in filling forms but we lost information by not recording details of medical history for each patient. Moreover, we did not record the severity and the stages of NDD for each patient. Therefore, we were not able to do subgroup analyses within AD or PD groups. A larger sample size with plenty of medical details is needed to allow discovering more knowledge from data. The pathophysiologic bases of the findings can be investigated in further and more basic biomedical research.
5. Conclusion
The current study showed that inpatients with AD have an increased risk for 28-day mortality from COVID-19. However, PD does not significantly predict higher mortality of COVID-19 infection compared with other hospitalized patients. Typically, patients with AD are elderly women and are more likely to have serious manifestations such as loss of consciousness on admission. They should be considered as high-risk patients and healthcare settings should be ready to provide more critical care for them such as early intubation and immediate O2 therapy.
Author contributions
MF contributed to the concept and interpretation of the results and helped with administrative affair. NMM and FT contributed to the design and to decision on eligibility, and carried out literature review. HM participated in designing the study, coordinated data record and performed statistical analyses. SZBJ guided development of the study protocol and contributed to the interpretation of the results and coordinated the research process. All the authors participated in drafting and its final approval.
Disclosure of interest
The authors declare that they have no competing interest.
Funding support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics code
Shahid Beheshti University of Medical Sciences with the ethics code of IR.SBMU.RETECH.REC.1399.499.
References
- 1.Jin J., Agarwala N., Kundu P., Harvey B., Zhang Y., Wallace E., et al. Individual and community-level risk for COVID-19 mortality in the United States. Nat Med. 2021;27(2):264–269. doi: 10.1038/s41591-020-01191-8. [DOI] [PubMed] [Google Scholar]
- 2.Markazi-Moghaddam N., Fathi M., Ramezankhani A. Risk prediction models for intensive care unit readmission: a systematic review of methodology and applicability. Auste Crit Care. 2020;33(4):367–374. doi: 10.1016/j.aucc.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager K.J., Kramer A., Chesnaye N.C., Couchoud C., Sanchez-Alvarez J.E., Garneata L., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei H., Yang Y., Zhou W., Zhang M., Shen Y., Tao D., et al. Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis. Lung Cancer. 2021;157:60–65. doi: 10.1016/j.lungcan.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emami A., Akbari A., Basirat A., Zare H., Javanmardi F., Falahati F., et al. The role of comorbidities on mortality of COVID-19 in patients with diabetes. Obes Med. 2021;100352 doi: 10.1016/j.obmed.2021.100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tessitore E., Carballo D., Poncet A., Perrin N., Follonier C., Assouline B., et al. Mortality and high risk of major adverse events in patients with COVID-19 and history of cardiovascular disease. Open Heart. 2021;8(1) doi: 10.1136/openhrt-2020-001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskandar E.N., Altschul D.J., de la Garza Ramos R., Cezayirli P., Unda S.R., Benton J., et al. Neurologic Syndromes Predict Higher In-Hospital Mortality in COVID-19. Neurology. 2021;96(11) doi: 10.1212/WNL.0000000000011356. e1527-e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J Alzheimers Dis. 2020;76(1):3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia X., Wang Y., Zheng J. COVID-19 and Alzheimer's disease: how one crisis worsens the other. Transl Neurodegener. 2021;10(1):15. doi: 10.1186/s40035-021-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Jimenez P., Munoz-Garcia M.I., Seoane D., Roca-Rodriguez L., Garcia-Reyne A., Lalueza A., et al. Cognitive impairment is a common comorbidity in deceased COVID-19 patients: a hospital-based retrospective cohort study. J Alzheimers Dis. 2020;78(4):1367–1372. doi: 10.3233/JAD-200937. [DOI] [PubMed] [Google Scholar]
- 13.Brown E.E., Kumar S., Rajji T.K., Pollock B.G., Mulsant B.H. Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer's disease and related dementias. Am J Geriatr Psychiatry. 2020;28(7):712–721. doi: 10.1016/j.jagp.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matias-Guiu J.A., Pytel V., Matias-Guiu J. Death rate due to COVID-19 in Alzheimer's disease and frontotemporal dementia. J Alzheimers Dis. 2020;78(2):537–541. doi: 10.3233/JAD-200940. [DOI] [PubMed] [Google Scholar]
- 15.Hwang J.M., Kim J.H., Park J.S., Chang M.C., Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci. 2020;41(9):2317–2324. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashim M.J., Alsuwaidi A.R., Khan G. Population risk factors for COVID-19 mortality in 93 countries. J Epidemiol Glob Health. 2020;10(3):204–208. doi: 10.2991/jegh.k.200721.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balci B., Aktar B., Buran S., Tas M., Donmez Colakoglu B. Impact of the COVID-19 pandemic on physical activity, anxiety, and depression in patients with Parkinson's disease. Int J Rehabil Res. 2021;44(2):173–176. doi: 10.1097/MRR.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artusi C.A., Romagnolo A., Ledda C., Zibetti M., Rizzone M.G., Montanaro E., et al. COVID-19 and Parkinson's disease: what do we know so far? J Parkinsons Dis. 2021;11(2):445–454. doi: 10.3233/JPD-202463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Y., Travaglio M., Popovic R., Leal N.S., Martins L.M. Alzheimer's and Parkinson's Diseases Predict Different COVID-19 Outcomes: A UK Biobank Study. Geriatrics (Basel) 2021;6(1) doi: 10.3390/geriatrics6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambergo-Michilot D., Barros-Sevillano S., Rivera-Torrejon O., De la Cruz-Ku G.A., Custodio N. Factors associated with COVID-19 in people with Parkinson’s disease: a systematic review and meta-analysis. Eur J Neurol. 2021:1–11. doi: 10.1111/ene.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearon C., Fasano A. Parkinson's disease and the COVID-19 pandemic. J Parkinsons Dis. 2021;11(2):431–444. doi: 10.3233/JPD-202320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Latapi P., Fearon C., Fasano A., Lang A.E. Parkinson's disease and COVID-19: do we need to be more patient? Mov Disord. 2021;36(2):277. doi: 10.1002/mds.28469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciaccio M., Lo Sasso B., Scazzone C., Gambino C.M., Ciaccio A.M., Bivona G., et al. COVID-19 and Alzheimer's disease. Brain Sci. 2021;11(3) doi: 10.3390/brainsci11030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirota G., Sato Y., Itoh D., Gonoi W., Hayashi T.Y., Sugita Y., et al. Pitfalls in chest CT findings of COVID-19 patients infected during hospitalisation. Clin Imaging. 2021;78:146–153. doi: 10.1016/j.clinimag.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173(4):262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 27.Fathi M., Markazi-Moghaddam N., Ramezankhani A. A systematic review on risk factors associated with sepsis in patients admitted to intensive care units. Austr Crit Care. 2019;32(2):155–164. doi: 10.1016/j.aucc.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Homauni A., Zargar Balaye Jame S., Hazrati E., Markazi-Moghaddam N. Intensive care unit risk assessment: a systematic review. Iranian journal of public health. 2020;49(8):1422–1431. doi: 10.18502/ijph.v49i8.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams H.J.A., Kwee T.C., Yakar D., Hope M.D., Kwee R.M. Systematic review and meta-analysis on the value of chest CT in the diagnosis of coronavirus disease (COVID-19): Sol Scientiae, Illustra Nos. AJR Am J Roentgenol. 2020;215(6):1342–1350. doi: 10.2214/AJR.20.23391. [DOI] [PubMed] [Google Scholar]
- 30.Trevisan K., Cristina-Pereira R., Silva-Amaral D., Aversi-Ferreira T.A. Theories of aging and the prevalence of Alzheimer's disease. Biomed Res Int. 2019;2019 doi: 10.1155/2019/9171424. 9171424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman M.A., Islam K., Rahman S., Alamin M. Neurobiochemical cross-talk between COVID-19 and Alzheimer's disease. Mol Neurobiol. 2021;58(3):1017–1023. doi: 10.1007/s12035-020-02177-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherbaum R., Kwon E.H., Richter D., Bartig D., Gold R., Krogias C., et al. Clinical profiles and mortality of COVID-19 inpatients with Parkinson's disease in Germany. Mov Disord. 2021;36(5):1049–1057. doi: 10.1002/mds.28586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignatelli L., Zenesini C., Belotti L.M.B., Baldin E., Bonavina G., Calandra-Buonaura G., et al. Risk of hospitalization and death for COVID-19 in people with Parkinson's disease or Parkinsonism. Mov Disord. 2021;36(1):1–10. doi: 10.1002/mds.28408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beatman E.L., Massey A., Shives K.D., Burrack K.S., Chamanian M., Morrison T.E., et al. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. 2015;90(6):2767–2782. doi: 10.1128/JVI.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]