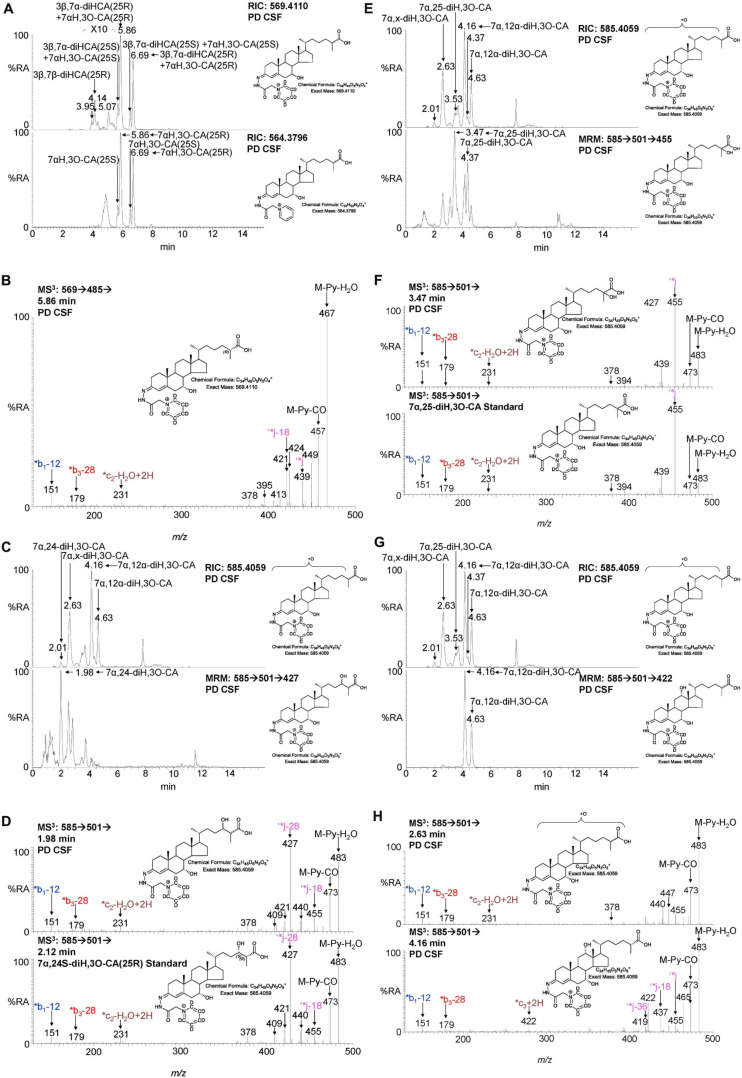
FIGURE 4.
LC-MS(MSn) analysis of cholestenoic acids found in CSF. (A) RIC of m/z 569.4110 ± 5 ppm revealing 3β,7β-diHCA and 3β,7α-diHCA (upper panel) and of 564.3796 ± 5 ppm revealing 7αH,3O-CA (lower panel). Each acid is present as two epimers, and each epimer gives syn and anti conformers of the GP-derivative. (B) MS3 (M+ → M+-Py →) spectrum of 3β,7α-diHCA(25R). (C,E,G) RIC of m/z 585.4059 ± 5 ppm revealing dihydroxy-3-oxocholestenoic acids (upper panels) and MRM-like chromatogram 585.4 → 501.3 → 427 highlighting 7α,24-diH,3O-CA (lower panel C), 585.4 → 501.3 → 455 highlighting 7α,25-diH,3O-CA (lower panel E) and 585.4 → 501.3 → 422 highlighting 7α,12α-diH,3O-CA (lower panel G). MS3 (M+ → M+-Py →) spectra of (D) 7α,24-diH,3O-CA in CSF (upper panel) and 7α,24S-diH,3O-CA(25R) authentic standard (lower panel), (F) 7α,25-diH,3O-CA in CSF (upper panel) and 7α,25-diH,3O-CA authentic standard (lower panel), and (H) 7α,x-diH,3O-CA (upper panel) and 7α,12α-diH,3O-CA (lower panel) in CSF.