Highlights
-
•
Male-based understanding of autism leads to poor understanding of autistic females.
-
•
We used a uniquely unbiased search strategy and quantitative evaluation of studies.
-
•
At least 3 well-powered studies identified sex/gender-modulation of autism effects.
-
•
No specific brain regions or networks show consistent sex/gender-modulating effects.
-
•
Future studies require larger sample sizes with more autistic female participants.
Keywords: Autism, Neurobiology, Neuroimaging, Brain, Sex, Gender
Abstract
Our current understanding of autism is largely based on clinical experiences and research involving male individuals given the male-predominance in prevalence and the under-inclusion of female individuals due to small samples, co-occurring conditions, or simply being missed for diagnosis. There is a significantly biased ‘male lens’ in this field with autistic females insufficiently understood. We therefore conducted a systematic review to examine how sex and gender modulate brain structure and function in autistic individuals. Findings from the past 20 years are yet to converge on specific brain regions/networks with consistent sex/gender-modulating effects. Despite at least three well-powered studies identifying specific patterns of significant sex/gender-modulation of autism-control differences, many other studies are likely underpowered, suggesting a critical need for future investigation into sex/gender-based heterogeneity with better-powered designs. Future research should also formally investigate the effects of gender, beyond biological sex, which is mostly absent in the current literature. Understanding the roles of sex and gender in the development of autism is an imperative step to extend beyond the ‘male lens’ in this field.
1. Introduction
Autism spectrum disorder (hereafter ‘autism’) is a neurodevelopmental condition characterized by early-onset and persistent difficulties in social communication and interaction along with repetitive or stereotyped behaviours (American Psychiatric Association, 2013). This condition is one with strikingly high heterogeneity as individuals diagnosed with autism may present with varying intensities of characteristics and difficulties across different domains pertaining to social, communication, behavioural, intellectual, and adaptive functioning and thus, autism is conceptualized as a ‘spectrum’ (Lai et al., 2013a). Despite the heterogeneity in the manifestation and intensity of symptoms, autism is consistently reported to disproportionately affect males (Ferri et al., 2018). Boys and men have been reported to be diagnosed four to five times more frequently than girls and women; however, population-based prevalence studies with active case-ascertainment suggest lower male-to-female ratios (3.25:1) as females who would meet the criteria for autism may be under-recognized clinically (Loomes et al., 2017).
1.1. Sex-bias and under-recognition of autistic females
There are a number of possibilities that can explain the under-recognition of autistic females. Firstly, the commonly referenced early descriptions of autism in the 1940s (Asperger, 1944, Kanner, 1943) were based on eight males and three females and another case series of four males only. Historical investigations have shown that there are even earlier accounts of autism in Europe that include female cases; however, these reports were overlooked by the field as they were not accessible in English until several decades later (Simmonds & Sukhareva, 2020). In addition to limited access to reports of autistic females, studies of characteristics and phenotypes of autism were largely derived from male individuals with this condition. With this ‘male-based’ understanding of autism, there is likely an ascertainment bias in the clinical recognition of this condition which may in part contribute to the apparent male bias in reported prevalence (Werling, 2016), despite the fact that the diagnostic conceptualization and broad-construct level definition of autism are meant to be sex/gender-independent (Lai et al., 2015). Further, females may present with partly different behavioural characteristics (Lai et al., 2015, Lai et al., 2017a, Mandy, 2017) that makes it more complicated for the autism phenotype to be recognized, and diagnosis to be made in a timely manner (Lai & Szatmari, 2020). In addition, gendered sociocultural contexts may further contribute to the under-recognition of autism in females (Dean et al., 2017, Kreiser and White, 2014).
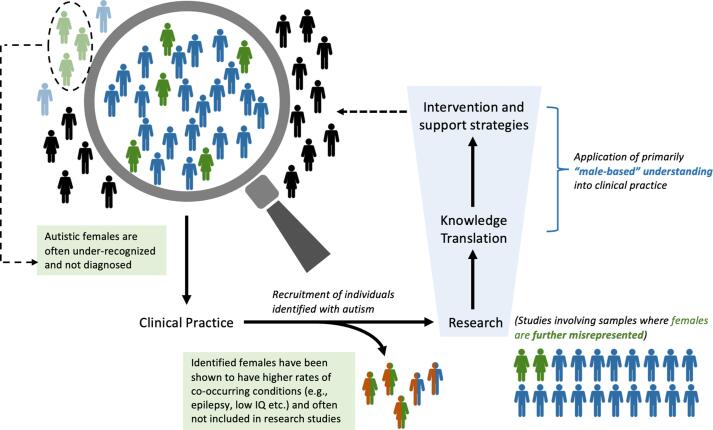
Autism research studies have been dominated by male participants. The underrepresentation of non-male participants is often due to small sample sizes which would limit the statistical power to detect small to moderate effects – only a handful of studies have attempted to directly address this issue by analyzing amalgamated large datasets (Kaat et al., 2021, Tillmann et al., 2018). Small sample sizes of non-male participants make it difficult to account for the effects of sex and gender, and thus researchers may limit their analyses to include only male participants (Jasmin et al., 2019, Ni et al., 2020, Prigge et al., 2021). In addition, clinically diagnosed autistic females are those who often present with coexisting behavioural, emotional, or cognitive difficulties (Duvekot et al., 2017, Dworzynski et al., 2012) and higher rates of co-occurring conditions, including epilepsy and low intelligence quotient, in comparison to autistic males (Lai et al., 2015). As researchers often screen their participants to maximize signal-to-noise ratio, the higher frequency of co-occurring conditions in autistic females makes them more likely to be excluded from research. As a result, the male-to-female participant ratio in research is even more exaggerated compared to the general population prevalence ratio. A number of meta-analyses (Hull et al., 2016, Philip et al., 2012, van Rooij et al., 2018, Via et al., 2011) have demonstrated a significantly exaggerated male-bias in autism research with large discrepancies between the population-based ratio (3.25:1) and those enrolled in brain morphological studies (∼6:1), task-based fMRI studies (∼15:1), and resting-state fMRI studies (∼9:1). These discrepancies leave autistic females significantly under-represented in research and poorly understood in practice, which further drives the male-biased knowledge base (Fig. 1).
Fig. 1.
The “Male-Lens” in Clinical Practice and Research in Autism. The male-based knowledge of autism and poor understanding of how autism presents in females is largely based on clinical practice and research primarily involving males given (1) the male predominance in prevalence and (2) females are often under-recognized and diagnosed individuals frequently present with co-occurring disorders (e.g., epilepsy) and low intelligence quotient and thus are more likely to be excluded from studies. This figure illustrates the concept of the “male-lens” in the field, which also largely reflects sex bias in autism since there is a lack of data pertaining to gender.
1.2. Autism neurobiology and sex/gender-modulation
Autism is a behaviourally defined condition based on the presence of difficulties with social communication and restricted/repetitive behaviours. Despite the complexity of the interactions of genetics and environment and phenotypic heterogeneity in behavioural manifestation, autism is fundamentally a condition of atypical neurodevelopment (Wolff et al., 2018). Current evidence suggests that autism is associated with variations in neural substrates including brain structure, functioning and connectivity (Ecker et al., 2015). For example, atypicalities in socioemotional processing have been associated with volumetric differences in frontotemporal regions and the amygdala and the presentation of repetitive and stereotypical behaviour has been associated with morphometric differences in the frontostriatal system (Langen et al., 2007, Lombardo et al., 2011). Clinically, these behavioural indicators seem to present differently in autistic males and females and therefore may have different phenotypes (Ecker, 2017). Studies so far have reported that the neurobiology of autism is potentially modulated by biological sex in quantitative as well as qualitative ways (Lai et al., 2017b); however, it is unclear whether there are specific brain regions or networks that consistently show these modulation effects. Although human neuroimaging studies seem to converge in support of atypical development and characteristics of the brain anatomy and functioning (Ecker et al., 2015), the patterns of sex and gender modulation in the neurobiology of autism still remain a significant knowledge gap.1
2. Research overview
The overarching goal of this systematic review is to synthesize what is known so far regarding the sex and/or gender modulation in the neurobiology of autism. Investigating how autism manifests differently in males and females may provide the key to understanding the sex-differential probability to and vulnerability in autism and also contribute to a better understanding of autistic females. The main questions of interest are: 1) what are the sex and gender differences in human autistic brains (beyond normative sex and gender differences), and 2) where are the key brain areas or networks that consistently show these differences. As such, we summarized findings of the available neuroimaging literature that reported sex or gender differences in brain structure or functioning associated with autism based on a sex- or gender-stratified examination of autism-control differences, or statistically comparing autism-control differences across sexes and genders by testing for sex/gender-by-diagnosis interactions. We also identified gaps in the literature and outlined important future considerations targeting the sex- and gender-based heterogeneity in autism.
3. Method
3.1. Search strategy
The systematic review was conducted in line with PRISMA guidelines (Moher et al., 2009) and registered with PROSPERO, identifier CRD42019138625. A health-science librarian (SB) developed the search strategy in consultation with coauthors. Four electronic databases (EMBASE, Medline, PsycINFO, and Web of Science) were searched for publications involving investigation of the modulating effects of sex or gender in the neurobiology of autism. The search was conducted using relevant subject headings and keywords for concepts of (‘sex’ OR ‘gender’) AND ‘autism’ AND ‘brain’. Keywords for ‘brain’ instead of ‘neuroimaging’ was used at this stage to capture all relevant studies investigating the underpinnings of the autistic brains to provide an overview of the current research. The search did not have limits to publication types or language, but it was limited to human studies and journal articles published from January 1, 2000 to March 15, 2021. The date restriction was applied to capture studies on the most current neuroimaging modalities. A focused grey literature search limited to conference papers and dissertations was conducted by searching three electronic databases (ProQuest Dissertations and Theses Global, Papers First, and Proceedings First). The references were managed with duplicates removed, using Mendeley (https://www.mendeley.com/). A copy of the search strategy is available in Appendix-Medline Search.
3.2. Search eligibility criteria
We implemented a two-tier screening procedure to adequately sort the identified studies. The first-level screened titles and abstracts for clear ineligibility (i.e., no mention of autism, animal studies, investigations of individuals with autistic traits but not an autism diagnosis, case studies, or literature reviews). For inclusion, the index of diagnosis was in accordance with the Diagnostic and Statistical Manual of Mental Disorder, 4th edition (DSM-IV) or International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) criteria (i.e., pervasive developmental disorders, PDD), or DSM-5 or ICD-11 criteria (i.e., autism spectrum disorder, ASD), and confirmed by the Autism Diagnostic Observation Schedule (ADOS/ADOS-2; Lord et al., 2000, Lord et al., 2012), Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003), Diagnostic Interview for Social and Communication Disorders (DISCO; Leekam et al., 2002), Childhood Autism Rating Scale (CARS; Schopler et al., 1988), or by a clinician. Studies that include male-only or female-only cohorts were deemed ineligible as they would provide no ground for comparison between sexes or genders. Studies that met this first tier of eligibility criteria at the level of the title/abstract screening were categorized by research subject to give an overview of the current research landscape. Specifically, the subject categories include: (1) brain structure and function, (2) behaviour and cognition, (3) genetics, (4) endocrinology, (5) environmental factors, (6) prenatal/perinatal factors, (7) immunology, (8) biochemistry, and (9) clinical trial/intervention. This overview provided a summary of the types of research that are available in the current literature that examine underpinnings of the autistic brains. Given our focus on neuroimaging, only studies under the ‘brain structure and function’ category moved forward as the second-tier criteria for full-text review. The screening was completed by two independent reviewers (KM and TS). In circumstances where there were discrepancies pertaining to whether a particular study met the eligibility criteria, a third reviewer (M-CL) provided an independent opinion. At this stage, studies were eligible for inclusion if:
-
1)The study’s research method used structural and/or functional brain imaging techniques, including:
-
•sMRI (structural magnetic resonance imaging)
-
•CT (computed tomography)
-
•DWI/DTI/DSI (diffusion weighted imaging; diffusion tensor imaging; diffusion spectrum imaging)
-
•fMRI (functional magnetic resonance imaging)
-
•PET (positron emission tomography)
-
•SPECT (single positron emission computed tomography)
-
•EEG (electroencephalography)
-
•MEG (magnetoencephalography)
-
•MRS (magnetic resonance spectroscopy)
-
•Brain stimulation techniques in combination with other brain imaging methods for functional brain mapping (e.g., TMS-EEG)
-
•
-
2)
They were empirical studies published in peer-reviewed journals;
-
3)
The study design compared autistic participants with typically developing ‘control’ individuals with both male and female participants in both groups; and
-
4)
The study examined sex and/or gender as a variable in their experimental design and analyses.
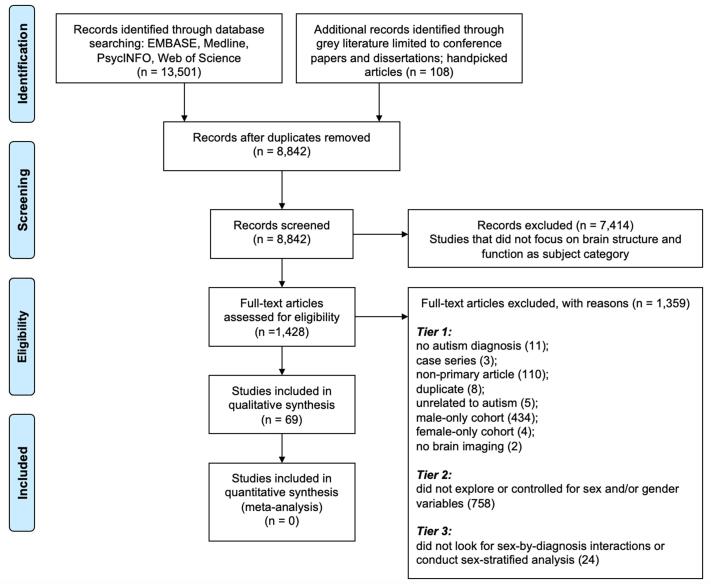
Articles were not included if they included male-only or female-only samples; if they did not examine sex or gender variables or controlled for sex and/or gender in the analyses (i.e., treated as covariates in the statistical models); or if the full-text article was not available or could not be accessed in English (Fig. 2).
Fig. 2.
PRISMA flow chart of study screening and selection process. This review focused on the qualitative synthesis for included studies since there was a limited scope for a meta-analysis with the high heterogeneity in study design, methodology, and neuroimaging modalities.
3.3. Data extraction and assessment
A standardized form (available upon request) was developed for data extraction from the primary studies, which included the following:
-
1)
Sample characteristics. The key information included sample size, whether the terms ‘sex’ and/or ‘gender’ were used and whether respective definitions were provided, participant sex and/or gender ratios, age range, criteria for autism diagnosis, co-occurring diagnoses, intelligence, ethnicity, socioeconomic status, and source of data (e.g., local recruitment of participants, use of open-source data).
-
2)
Study details. This included outcome measures of the study, imaging modality employed, methods used (i.e., region-of-interest, whole-brain approach), whether there were sex and/or gender matched controls, whether the variables ‘sex’ or ‘gender’ were adequately defined and measured, and how sex and/or gender variables were analyzed (e.g., sex/gender-by-diagnosis interaction, sex/gender-stratified analysis).
-
3)
Details of statistical analysis. This included reported effect size of significant brain findings, or data to calculate effect size using descriptive statistics for the dependent variable of interest (e.g., reported means and standard deviations).
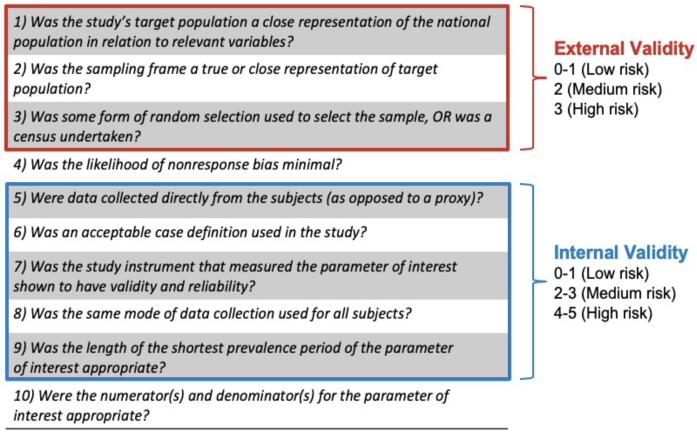
A modified version of the Hoy Risk of Bias Assessment Tool (Fig. 3; Hoy et al., 2012) was used to evaluate included studies. There were two items pertaining to nonresponse bias and numerators/denominators of the parameter of interest that were not applicable for this systematic review. Thus, the modified tool provided separate summary scores representing the risk of bias for external validity and internal validity, respectively. For evaluation of external validity based on items 1–3, a summary score of 0–1 indicated a low risk; 2 a moderate risk; and 3 a high risk of bias. For evaluation of internal validity based on items 5–9, a summary score of 0–1 indicated a low risk; 2–3 a moderate risk; and 4–5 a high risk of bias. All studies were independently rated by KM and TS and confirmed by M-CL for any discrepancies. Inter-rater reliability was measured based on percent agreement between the two raters.
Fig. 3.
Risk of Bias Assessment. A modified version of the Hoy Risk of Bias Assessment Tool (Hoy et al., 2012) was used to evaluate included studies. There were two items pertaining to nonresponse bias and numerators/denominators of the parameter of interest that were not applicable for this systematic review. Thus, the modified tool provided separate summary scores representing the risk of bias for external validity and internal validity, respectively.
3.4. Statistical evaluation
The studies that were included were very heterogeneous in terms of design, methodology, neuroimaging modalities used and sample sizes. There was a lack of consistency in the outcome measures and age group of participants across studies within each imaging modality. This heterogeneity generated a very limited scope for a quantitative meta-analysis and therefore, it was not conducted. However, to characterize the current literature, a decision tree was performed and visualized using the R rpart and rpart.plot packages that implement CART (classification and decision trees) to examine features of studies that reported ‘positive’ (i.e., statistically significant) findings against those that reported ‘negative’ (i.e., null) findings with regard to any sex/gender-modulation effects. Studies that reported positive findings were coded ‘1’ and those reporting negative findings were coded ‘0’. This binary variable assigned as the ‘significance’ was predicted based on study features including total sample size, male-to-female participant ratio for total sample, male-to-female participant ratio for autism sample, method (i.e., region-of-interest, whole brain), and imaging modality. Across several study features, this list enabled the largest number of studies to remain in this analysis. Other study features of interest, including IQ and age range of participants, were not available for many studies and thus were not included. The decision tree was analyzed for the study features that best predicted the ‘significance’ of study findings (i.e., whether a positive finding was reported).
For each imaging modality, the studies with the largest total sample size and/or largest autistic female sample size that reported positive findings were further examined with the results qualitatively summarized. In cases where the largest study from an imaging modality did not have the largest autistic female sample size, the two studies were both examined, respectively. A sensitivity power analysis was conducted to evaluate whether the effect sizes reported in the studies would be reasonably found given the studies’ sample sizes. The minimally detectable effect (MDE) was calculated using the R pwr package based on sample size, probability of making a type I error (α) as 0.05, and power of 0.80. The MDE for each study was then compared with the reported effect sizes where necessary information was available. The MDE and reported effect sizes were critically analyzed for potentially exaggerated effect sizes and false positives in these studies (Buxbaum et al., 2019). Potential overlap in participants from studies with data derived from common data repositories could not be ascertained due to lack of access to original individual-level data.
4. Results
4.1. Overview of current research
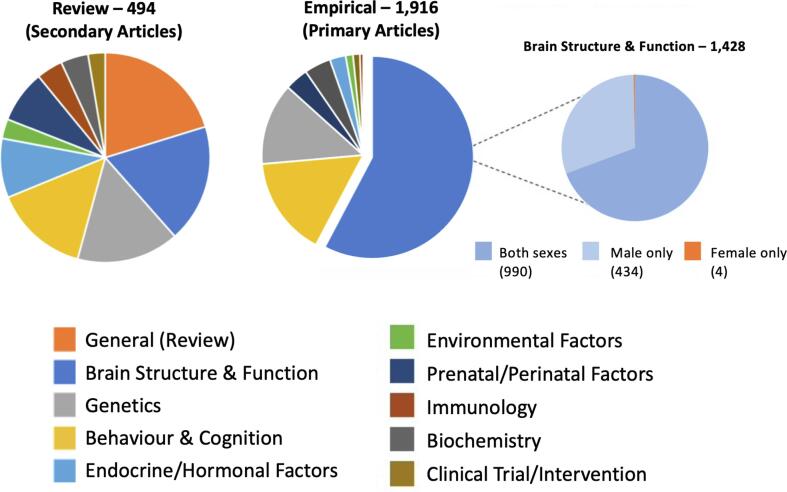
The systematic search generated a total of 13,609 articles (including grey literature and handpicked articles, N = 108). After removal of duplicates, 8,842 unique citations were screened for relevance and categorized by subject to generate an overview of the current research landscape (Fig. 4). Focusing on the ‘Brain Structure & Function’ category (N = 1,428), 30% of these studies examined male-only participants (N = 434) and female-only participants (N = 4). Such discrepancy (434:4) provides no ground for comparison for the findings between male-only and female-only studies, thus these studies were excluded, leaving 990 studies for full-text review. At this stage, 77% of these studies (N = 758) were excluded because sex and/or gender variables were not examined or treated as a covariate in analysis; only 93 studies had some consideration for sex and/or gender variables. Of the 93 studies, 69 studies that examined sex/gender-by-diagnosis interaction effects and/or sex/gender-stratified analyses of autism-control differences were eligible for systematic review and were retained for qualitative synthesis (Alaerts et al., 2016, Andrews et al., 2017, Andrews et al., 2019, Andrews et al., 2021, Beacher et al., 2012a, Beacher et al., 2012b, Bedford et al., 2020, Bletsch et al., 2021, Bosco et al., 2019, Contarino et al., 2016, Di and Biswal, 2016, Doyle-Thomas et al., 2014, Floris et al., 2021, Fung et al., 2021, Giuliano et al., 2018, Guo et al., 2019, Hammill et al., 2021, Henry et al., 2018, Hernandez et al., 2020, Holt et al., 2014, Hsu et al., 2018, Irimia et al., 2017, Irimia et al., 2018, Kirkovski et al., 2015, Kirkovski et al., 2016a, Kirkovski et al., 2016b, Kirkovski et al., 2018, Kirkovski et al., 2020, Lai et al., 2013b, Lai et al., 2019b, Laidi et al., 2017, Lawrence et al., 2020b, Lee et al., 2020, Lee et al., 2021, Lei et al., 2019, Lin et al., 2019, Maier et al., 2018, Mei et al., 2020, Mitelman et al., 2018, Moessnang et al., 2020, Nordahl et al., 2020, Olafson et al., 2021, Olson et al., 2020, O’Neill et al., 2020, Peterson et al., 2019, Postema et al., 2019, Retico et al., 2016, Saunders et al., 2016, Schaer et al., 2015, Schneider et al., 2013, Schumann et al., 2010, Shen et al., 2018, Smith et al., 2019, Subbaraju et al., 2017, Sussman et al., 2015, Tomasi and Volkow, 2019, Trakoshis et al., 2020, van Rooij et al., 2018, Williams et al., 2020, Yang and Lee, 2018, Yankowitz et al., 2020, Yoshimura et al., 2021, Ypma et al., 2016, Zeestraten et al., 2017, Zhang et al., 2018, Bode et al., 2011, Ecker, 2019, Kozhemiako et al., 2020, Lawrence et al., 2020a; Appendix-Table 1). Data extracted from these studies were stratified by neuroimaging modality, including structural MRI, CT, DWI/DTI/DSI, task fMRI, resting-state fMRI, PET, resting-state EEG, MEG, MRS, TMS-EEG, as well as different methods of analysis of sex and/or gender variables.
Fig. 4.
Overview of autism neuroscience research. At the level of the title/abstract screening stage, articles were first divided into primary and secondary studies and categorized for research subject to provide an overview of the current research landscape. This demonstrates a significant portion of studies that used study cohorts of only male participants and only four studies that used a cohort of only female participants.
4.2. Study definitions of ‘sex’ versus ‘gender’
The current neuroimaging literature examining sex/gender modulating effects on brain structure and function in autism does not provide a clear differentiation between the terms, ‘sex’ and ‘gender’ (Table 1). There were only two studies that clearly indicated male/female groups were assigned based on parent-report of (biological) sex designated at birth. For all other studies, it was unclear whether there were any proxy measures for sex and/or gender. Of the 69 included neuroimaging studies, 19 studies correctly specify that ‘sex’ refers to biological sex. The remaining 50 studies include those that use the term ‘sex’ with no definition provided (N = 38) and those that use the term ‘gender’ with no definition provided (N = 12). In addition to a lack of clear definitions for the use of ‘sex’ and ‘gender’ terms for a large majority of included studies, there were no studies that implemented measures or investigated the effects of sex and gender variables separately. As such, the studies that did report significant ‘sex’ or ‘gender’-modulating effects are likely reflecting the potential modulating effects of both sex and gender; therefore, the term ‘sex/gender’ is used hereafter in this article.
Table 1.
Summary of ‘sex’ and ‘gender’ definitions.
| N | Definition provided? | Where a definition was provided, was the term defined/used correctly? | Proxy measure provided for sex and/or gender? | |
|---|---|---|---|---|
| Studies using ‘sex’ term |
57 | 33.3% Yes (N = 19) 66.7% No (N = 38) |
100% Yes (N = 19) | 3.5% Yes (N = 2); participants were assigned to the female/girl or male/boy group based on parent-report of biological sex designated at birth 96.5% No (N = 55) |
| Studies using ‘gender’ term |
12 | No (12, 100%) | N/A – no definition provided | None for all studies |
4.3. Assessment for risk of bias
A risk of bias assessment of included studies revealed high inter-rater reliability agreement (0.96, 95% CI 0.91–0.98) between reviewers. All studies were rated a score of 3, indicating high risk for external validity with low generalizability of findings since the participants were often recruited locally from hospitals and communities or the neuroimaging data were leveraged from data sharing initiatives. These included studies involving autistic individuals that were not ascertained by random sampling from the general population and were therefore unlikely to be representative of the population of autistic individuals at large. On the other hand, the risk of bias scores for internal validity ranged from 0 to 1, indicating low risk, and the most common risk was for studies that did not use the same approach for data collection for all participants. Such risk was identified for studies leveraging neuroimaging data from large data sharing initiatives where there were multiple contributing sites.
4.4. Characteristics of studies that reported significant versus non-significant findings on any sex/gender-modulation effects
From the 69 neuroimaging studies, 44 studies reported significant findings from analysis of sex/gender-by-diagnosis interactions and/or sex/gender-stratified analysis of autism-control differences and 25 studies reported non-significant findings (Table 2).
Table 2.
Comparison of studies reporting positive/significant and negative/non-significant findings with regard to sex/gender-modulation effects based on neuroimaging modality and study sample.
| Studies reporting positive/significant findings (N = 44) | Studies reporting negative/non-significant findings (N = 25) | |
|---|---|---|
| Number of studies (N/Ntotal) by imaging modality (i.e., DTI/DWI/DSI, MEG, MRS, PET, rs-EEG, rs-fMRI, sMRI, task-fMRI, TMS-EEG) |
DTI/DWI/DSI (8/11) MRS(2/4) rs-fMRI (13/15) sMRI(15/27) task-fMRI (6/8) |
DTI/DWI/DSI (3/11) MEG (1/1) MRS (2/4) PET(1/1) rs-EEG (1/1) rs-fMRI (2/15) sMRI (12/27) task-fMRI (2/8) TMS-EEG (1/1) |
| Sample size, Ntotal: Mean ± standard deviation Median (IQR) Range |
371.68 ± 617.34 171 (111.75) 49–3607 |
391.32 ± 679.67 126 (3 7 3) 25–3222 |
| Average M:F participant ratio for whole sample (N of male to ONE female, mean ± standard deviation) |
1.89 ± 1.28 |
2.46 ± 1.46 |
| Average M:F participant ratio for autism group (N of male to ONE female, mean ± standard deviation) |
2.32 ± 1.82 | 3.55 ± 2.81 |
| Average M:F participant ratio for control group (N of male to ONE female, mean ± standard deviation) |
1.64 ± 1.06 | 2.11 ± 1.68 |
The decision tree (Fig. 5) revealed that total sample size was the most important feature with the percentage of variable importance as follows: 57% total sample size, 22% male-to-female participant ratio (autism group), 16% male-to-female participant ratio (total sample), 6% imaging modality, and 0% method (ROI, whole-brain). Descriptive statistics of the two most important continuous variables, total sample size and male-to-female participant ratio are presented in Fig. 6, Fig. 7, respectively.
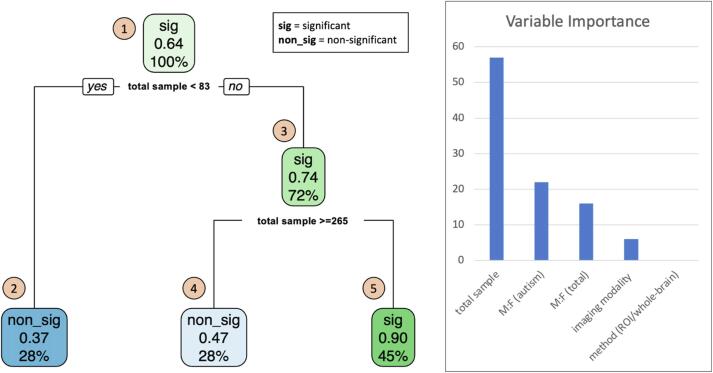
Fig. 5.
Decision tree – exploring the most important factor associated with the significance of reported sex/gender-by-diagnosis interaction effects and sex/gender-stratified autism-control differences from studies reviewed (N = 69). Findings from studies are coded by a binary variable, ‘significance’, where positive/significant findings are coded ‘1’ and negative non-significant findings are coded ‘0’ and expressed as a function of study features included total sample size, male-to-female participant ratio (total sample), male-to-female participant ratio (autism group), method (i.e., region-of-interest, whole-brain), and imaging modality. At the top of the decision tree, (1) the proportion of studies that reported a significant finding is 64%. (2) The first node asks whether the total sample size of the study is less than 83. 28% of the studies include a total sample size less than 83 where the probability of reporting a significant finding is 37%. (3) 72% of the studies include a total sample size greater than or equal to 83 where the probability of reporting a significant finding is 74%; however, (4) this probability decreases to 47% if the total sample size is greater than or equal to 265 and (5) increases to 90% if the total sample size is less than 265.
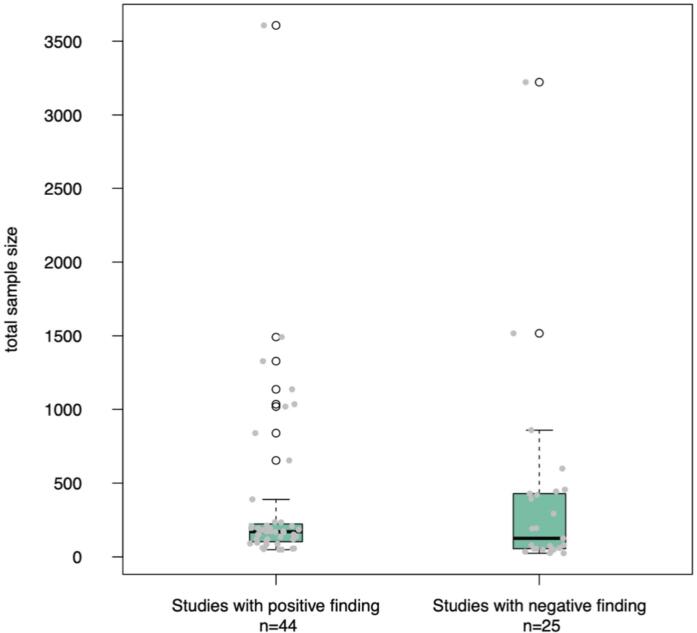
Fig. 6.
Distribution of total sample size for studies (N = 69) with positive versus negative findings pertaining to sex/gender-by-diagnosis interaction effects and sex/gender-stratified autism-control differences. The distribution of total sample size is shown here via box-and-whisker plots overlaid with individual study data points for all studies, with shaded markers representing individual studies and unshaded circles (of the box-and-whisker plots) indicating outliers. It is important to note that the large majority of studies with a total sample size greater than or equal to N = 234 are multi-site studies with the exception of four studies (Hammill et al., 2021, N = 839; Lee et al., 2020, N = 429; Nordahl et al., 2020, N = 420; Shen et al., 2018, N = 236) where the data were obtained at a single site.
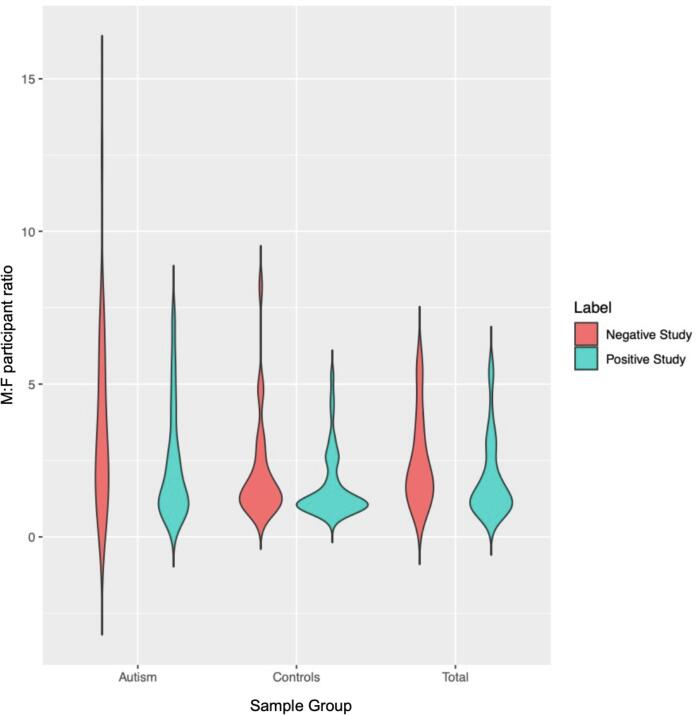
Fig. 7.
Distribution of male-to-female participant ratio for studies (N = 69) that reported positive versus negative findings pertaining to sex/gender-by-diagnosis interaction effects and sex/gender-stratified autism-control differences. The distribution of male-to-female participant ratio is shown here for all autism groups, control groups, and overall sample (total). The neuroimaging studies that reported a negative/non-significant finding appear to have larger male-to-female participant ratio with much greater spread especially for the autism group in comparison to the studies that reported positive/significant finding.
4.5. Notable findings of the available neuroimaging literature
Due to high heterogeneity in study design and participant demographics, the findings of very few studies could be directly compared. Further, there was no clear consensus for specific brain regions or networks showing consistent sex/gender-modulating effects. Findings from the available literature involve several different imaging modalities. Generally, certain imaging techniques (e.g., T1-weighted MRI, CT, and DWI/DTI/DSI) can provide inferences for brain structure while others can provide inferences for brain function (e.g., fMRI, MEG, and EEG); however, this distinction is not always clear. For example, MRI techniques including Arterial Spin Labelling informs perfusion which can provide both structural and functional implications. In addition, MRS and PET address molecular-level mechanisms that may underlie both structure and function. Thus, to minimize this potential ambiguity, findings are presented by imaging modality.
4.5.1. Diffusion imaging
There were eight studies of white matter connectivity that examined sex/gender-by-diagnosis interaction effects and/or sex/gender-stratified analysis of autism-control differences with significant findings (Table 3). One notable finding was the right inferior fronto-occipital fasciculus (IFOF) where two studies (N = 53, Bode et al., 2011; N = 213, Zeestraten et al., 2017) reported significant autism-control differences when stratified by sex/gender; however, the direction of findings differed as one reported greater fractional anisotropy (FA) in teenage autistic males compared to typically developing teenage males and the other reported lower FA in adult autistic males compared to typical adult males. Neither of these two studies found significant autism-control differences in FA among female participants. Lei et al. (2019), on the other hand, reported significant, widespread bilateral reductions in FA in association tracts, including the IFOF; however, this reduction was only found in autistic females and there were no reported significant differences in the male groups in sex/gender-stratified analyses (N = 120). In a unique sample of preschool aged-children, increased FA in several commissural, projection, and association tracts, including the IFOF, was found in both autistic males and females compared to typically developing controls; however, a sex-by-diagnosis interaction was only observed in measures of axial diffusivity (AD) in clusters including areas of the body, genu, and splenium of the corpus callosum with autistic females showing increased AD and autistic males showing decreased AD compared to typically developing controls (N = 181, Andrews et al., 2019). To date, this is the largest diffusion-weighted imaging study of autistic preschool-aged children.
Table 3.
Significant findings of investigations of sex/gender-by-diagnosis interactions and sex/gender-stratified analysis of autism-control differences in white matter connectivity (DTI/DWI/DSI).
| Study | Country of origin for study sample | Method (‘sex’ or ‘gender’ is based on the term used in the study) | Metrics/Outcome measure | Sample size (M:F) | Age range | Covariates in analysis | Notable results (‘sex’ or ‘gender’ is based on the term used in the study) |
|---|---|---|---|---|---|---|---|
| Bode et al., 2011 | Finland | DTI (whole-brain) gender stratified |
FA, MD | 27 autism (20:7) 26 TD (17:9) |
Autism11.4–17.6 yr TD 11.7–17.3 yr |
None reported | Greater FA in the area containing clusters of optic radiation and the right IFOF (significant in males only when grouped by gender) |
| Beacher et al., 2012b | United Kingdom | DTI; sMRI (ROIs: CC-genu, body, splenium; CING, CST, SLF, CR, MCP) sex*dx |
FA, MD, whole-brain volume | 28 autism (15:13) 30 TD (15:15) |
no range; autism (M) 32 ± 10 yr autism (F) 32 ± 7 yr TD (M) 28 ± 8 yr TD (F) 32 ± 8 yr |
NART score (a proxy for overall intellectual function) | significant sex*dx interaction in total white matter volume, regional gray matter volume in the right parietal operculum, and FA in the body of CC, CING, and CR |
|
Irimia et al., 2017 (GENDAAR) |
United States |
DWI; sMRI (whole-brain) sex*dx |
GM thickness, volume, cortical area, mean curvature, CD |
110 autism (55:55) 83 TD (43:40) |
autism 7–18 yr TD 8–18 yr |
none reported |
significant sex*dx interaction in white matter CD innervating, bilaterally, the lateral aspect of the temporal lobe, temporo-parieto-occipital junction and the medial parietal lobe |
|
Zeestraten et al., 2017 (MRC-AIMS) |
United Kingdom |
DTI (ROIs: frontal fiber bundles – anterior segment of AF, long segment of AF, cingulum, uncinate, IFOF and two non-frontal fiber tracts – posterior segment of AF, ILF) sex*dx + sex stratified |
FA |
98 autism (61:37) 115 TD (61:54) |
autism (M) 18–41 yr autism (F) 18–37 yr TD (M) 18–45 yr TD (F) 18–52 yr |
scanning centre, age, FSIQ |
significant sex*dx interaction in frontal tracts only; non-frontal tracts revealed no interaction lower tract mean FA in autism group compared in TD in all frontal tracts except long segment of right AF and all investigated non-frontal tracts (significant in males only) |
|
Lei et al., 2019 (GENDAAR) |
United States |
DTI (whole-brain) sex stratified |
FA |
81 autism (56:25) 39 TD (23:16) |
autism 4–21 yr TD 5–18 yr |
none reported |
significant widespread bilateral reductions in FA in association tracts (CING, IFOF, ILF, SLF, and UF), projection (ATR, CST), commissural fibers (FMAJ, FMIN) in autistic subjects (significant in females only) |
|
Andrews et al., 2019 |
United States |
DWI (whole-brain) sex*dx |
FA, MD, RD, AD |
127 autism (85:42) 54 TD (28:26) |
autism 2.2–4.1 yr TD 2.1–4.1 yr |
age, relative movement |
sex*dx interaction in measures of AD across six significant clusters incorporating areas of the body, genu, and splenium of CC; females (ASD > TD), males (ASD < TD) |
|
Kirkovski et al., 2020 |
Australia |
DWI (whole-brain) sex stratified |
FD, FC, FDC |
25 autism (12:13) 24 TD (12:12) |
autism 21–55 yr TD 19–56 yr |
framewise displacement |
FDC at the CC (posterior midbody/isthmus) was significantly reduced for females with ASD compared TD females no differences found between males with ASD and TD males |
|
Bletsch et al., 2021 (MRC-AIMS) |
United Kingdom |
DTI (whole-brain) sex*dx sex stratified |
FA, MD, GWC at GWM boundary, different sampling depths within superficial WM and into GM |
92 autism (53:39) 92 TD (51:41) |
all participants 18–52 yr |
age, FSIQ |
significant sex*dx interactions for FA and MD (most pronounced within the superficial WM) no sex*dx interaction effects for GWC sex-stratified results in males mainly showed reduction in FA (ASD < TD) and increased MD (ASD > TD) that was most pronounced effects at the GWM boundary, −1 mm and −2 mm below GWM boundary sex stratified results in females mainly showed increased FA (ASD > TD) and a reduction in MD (ASD < TD) that was most pronounced effects at GWM boundary and at 30% and 60% cortical thickness GWC was reduced (ASD < TD) in both ASD males and females compared to same-sex counterparts at 30% cortical thickness |
Abbreviations: ABIDE, Autism Brain Imaging Data Exchange; AD, axial diffusivity; AF, arcuate fasciculus; ATR, anterior thalamic radiation; CC, corpus callosum; CD, connectivity density; CING, cingulum; CR, corona radiata; CST, corticospinal tract; FA, fractional anisotropy; FC, fiber cross-section; FD, fiber density; FDC, fiber density and cross-section; FMAJ, forceps major; FMIN, forceps minor; FSIQ, full scale intelligence quotient; GENDAAR, Gender Exploration of Neurogenetics and Development to Advance Autism Research; GM, gray matter; GWC, gray-white matter tissue contrast; GWM, gray-white matter; IFOF, interior frontal occipital fasciculus; ILF, inferior longitudinal fasciculus; MCP, middle cerebellar peduncle; MD, mean diffusivity; MRC-AIMS, Medical Research Council Autism Imaging Multicentre Study; NART, National Adult Reading Test; RD, radial diffusivity; ROI, region-of-interest; SLF, superior longitudinal fasciculus; UF, uncinate fasciculus
4.5.2. Magnetic resonance spectroscopy
Of the four MRS studies that examined sex/gender-by-diagnosis interaction effects and/or sex/gender-stratified analysis of autism-control differences, there were two studies (N = 174, O’Neill et al., 2020; N = 57, Fung et al., 2021) that reported significant findings (Appendix-Table 1). Using a region-of-interest approach focusing on the bilateral thalami and left dorsolateral prefrontal cortex, Fung and colleagues (2020) examined concentrations of GABA and reported a significant gender-by-diagnosis interaction in left dorsolateral prefrontal cortex. On the other hand, using a whole-brain approach, O’Neill et al. (2020) examined concentrations of N-acetyl compounds, glutamate and glutamine, creatine and phosphocreatine, as well as choline compounds and reported a bilateral sex-by-diagnosis interaction in the posterior thalamic radiations and the left centrum semiovale that was strongest for glutamate + glutamine and N-acetyl compounds. This study had the largest total sample size compared to the MRS studies that did not report significant sex/gender-modulating effects (N = 36, Doyle-Thomas et al., 2014; N = 26, Kirkovski et al., 2018).
4.5.3. Resting-state and task fMRI
There were 13 resting-state fMRI (Table 4) and six task fMRI studies (Table 5) that reported significant sex/gender-by-diagnosis interaction effects and/or autism-control differences in sex/gender-stratified analysis. Among these studies, there was substantial variability in study design and brain measures. Resting-state fMRI studies involved different analysis approaches examining whole-brain and different regions/networks-of-interest. Despite the variability in brain measures, there were eight resting-state fMRI studies that specifically examined sex/gender-by-diagnosis interaction effects. Five studies, including one with two replication samples (N = 234, Alaerts et al., 2016; N = 1,019 discovery sample, N = 309 replication sample 1 of 2, Floris et al., 2021; N = 168, Smith et al., 2019; N = 135, Trakoshis et al., 2020; N = 96, Yang & Lee, 2018), reported disordinal (cross-over) interactions, which implies substantial autism-control differences that are different in directionality across sex/gender.
Table 4.
Significant findings of investigations of sex/gender-by-diagnosis interactions and sex/gender-stratified analysis of autism-control differences in resting-state functional connectivity (rs-fMRI).
| Study | Country of origin for study sample | Method (‘sex’ or ‘gender’ is based on the term used in the study) | Metrics/Outcome measure | Sample size (M:F) | Age range | Covariates in analysis | Notable results (‘sex’ or ‘gender’ is based on the term used in the study) |
|---|---|---|---|---|---|---|---|
| Alaerts et al., 2016 (ABIDE) | International | rs-fMRI (whole-brain region-to-region functional connectivity explored with whole-brain parcellated network of 200 ROIs) sex*dx |
resting-state functional connectivity (seed-to-voxel, whole-brain region-to-region) |
84 autism (42:42) 150 TD (75:75) |
all female participants 7–30 yr all male participants matched pair-wise for age and IQ |
frame-wise displacement scores, site, FSIQ, age |
seed-to-voxel significant sex*dx effects for right STS-seed, left STS-seed and PCC-seed whole-brain ROI-to-ROI significant sex*dx effects for right SFG-left MTG connection and right SFG-precuneus/PCC connection males (ASD < TD) females (ASD > TD) |
|
Ypma et al., 2016 (CFSA - primary; ABIDE - replication) |
United Kingdom; International |
rs-fMRI (ROIs: DMN defined as 58 8 mm-radius spherical ROIs derived from meta-analysis of fMRI studies) sex stratified |
a functional DMN intra-connectivity (density of all binary intra-DMN edges minus a constant number of such edges expected in a random network) |
CFSA 51 autism (35:16) 40 TD (20:20) ABIDE 463 autism (408:55) 517 TD (428:89) |
CFSA all participants 12–18 yr ABIDE all participants 6–58 yr (47% in 12–18 yr range) |
site, age, IQ, mean frame-wise displacement |
significant reduction in DMN intra-connectivity in both males and females with ASD compared to same-sex controls (significant reduction in DMN intra-connectivity was replicated in ABIDE sample) |
|
Subbaraju et al., 2017 (ABIDE) |
International |
rs-fMRI (ROIs: 90 regions of the brain based on AAL atlas) gender stratified |
temporal signals and spatial distribution weights from projection matrix of BOLD time-series signals |
505 autism (443:62) 530 TD (435:95) |
all participants 6.5–58 yr |
none reported |
regional differences in resting state activities: autistic males showed a clear shift in activities to PFC; diminished activities in other parts of the brain compared to TD males autistic females showed diminished activities in posterior and medial portions compared to TD females |
|
Yang and Lee, 2018 (ABIDE) |
International |
rs-fMRI (ROIs: four mentalizing regions – mPFC, bilateral TPJ, precuneus) sex*dx |
intrinsic functional connectivity (average BOLD time course extracted from each seed region correlated with time courses of all voxels in the rest of the brain) |
48 autism (24:24) 48 TD (24:24) |
no range; autism (M) 14.5 ± 4.7 yr autism (F) 14.4 ± 4.6 yr TD (M) 14.9 ± 4.3 yr TD (F) 14.5 ± 4.7 yr |
age, IQ scores, eye status during scanning, site information (TR, voxel size, length of scan) |
sex*dx interaction was found in both short- and long- distance functional connectivity effects autistic males showed overconnectivity (ASD > TD) in the bilateral TPJ autistic females showed underconnectivity (ASD < TD) in mPFC, precuneus, right temporo-parietal region |
| Tomasi and Volkow (2019) (ABIDE) | International | rs-fMRI (whole-brain + whole thalamic partition as seed region for seed-voxel correlation analyses) sex stratified |
lFCD, LI, ALFF, seed-voxel correlation maps | 656 autism (565:91) 835 TD (602:233) |
all participants 7–40 yr |
age, FSIQ, mean frame-wise displacement | autistic males showed lower lFCD in the anterior thalamus compared to TD males; group differences in thalamic lFCD between autistic females and typically developing females were not statistically significant |
| Smith et al., 2019 | United States | rs-fMRI (whole-brain) sex*dx | global functional connectivity in cortico-cerebellar organization | 79 autism (56:23) 89 TD (65:24) |
autism 11–62 yr TD 10–54 yr |
age, frame-wise displacement, global correlation level (GCOR) | two clusters in bilateral cerebellum with sex*dx interaction in global connectivity males showed cortico-cerebellar hypoconnectivity (ASD < TD) females showed cortico-cerebellar hyperconnectivity (ASD > TD) |
|
Lee et al., 2020 |
United States |
rs-fMRI (ROIs: amygdala connectomes) sex*dx sex*dx*age |
amygdala resting-state functional connectivity map (multivariate distance matrix regression; univariate analysis) |
116 autism (80:36) 58 TD (31:27) |
all participants 2–7 yr |
none reported |
significant sex*dx interaction observed for left amygdala for multivariate distance matrix regression model; four sex*dx interaction clusters (left amygdala, left DMPFC, left ventral PFC, left lingual gyrus, between right amygdala and right poster cingulate cortex) |
|
Hernandez et al., 2020 (GENDAAR) |
United States |
rs-fMRI (ROIs: bilateral NAcc – correlated with every other voxel in the brain to generate functional connectivity maps) genetic risk*sex*dx |
reward network resting-state functional connectivity (with additive impact of genetic risk – ASD-associated OXTR variants) |
87 autism (37:50) 86 TD (34: 52) |
all participants 8–17 yr |
MRI data collection site, IQ, number of functional volumes remaining after motion scrubbing |
sex significantly modulated the relationship between OXTR genetic risk and NAcc connectivity in the ASD group only. Relative to their male counterparts, as genetic risk for ASD increased, females with ASD showed significantly greater connectivity between the NAcc and regions of the mesolimbic reward system, including the caudate, pallidum, and putamen, as well as bilateral thalamus, right prefrontal cortex, and left medial prefrontal cortex |
|
Lawrence et al., 2020a (GENDAAR) |
United States |
rs-fMRI (whole-brain and ROIs: SN, DMN, CEN) sex*dx + sex stratified |
within- and between-network functional connectivity of SN, DMN, and CEN |
80 autism (34:46) 89 TD (41:48) |
no range; autism (M) 13.32 ± 3.04 yr autism (F) 13.50 ± 2.52 yr TD (M) 13.71 ± 2.64 yr TD (F) 13.15 ± 3.04 yr |
general cognitive ability, pubertal development, site/scanner |
whole-brain functional connectivity (sex stratified): female ASD group displayed no significantly atypical patterns of connectivity; male ASD group exhibited atypical SN connectivity whole-brain functional connectivity (sex*dx): significant sex*dx interaction observed in SN connectivity with left posterior parietal cortex and precuneus ROI-based network functional connectivity (sex stratified): female ASD group exhibited increased positive connectivity between the DMN (PCC) and CEN (L PPC); male ASD group displayed less positive connectivity with the CEN (R DLPRC with R PPC) ROI-based network functional connectivity (sex*dx): sex*dx interaction between the DMN and the CEN, in the within- and between-network connectivity of the SN (did not attain statistical significance after correction for multiple comparisons) |
|
Kozhemiako et al., 2020 (ABIDE) |
International |
rs-fMRI (ROIs: seven network mask of cerebellum – visual, somatomotor, dorsal attention, ventral attention, limbic, fronto-parietal control, DMN) sex stratified |
local connectivity quantified as regional homogeneity (ReHo) – concordance of time-series of neighbouring voxels |
194 autism (102:92) 196 TD (104:92) |
all participants 6–26 yr |
none reported |
increases in local connectivity in participants with ASD in the somatomotor and limbic networks and decreased local connectivity within the default mode network – alterations were more pronounced in females with ASD ASD(M): decreased local connectivity in ventral attention and DMN compared to TD males ASD(F): decreased local connectivity in ventral attention, frontoparietal control, DMN; increased local connectivity in limbic network compared to TD females |
|
Trakoshis et al., 2020 (MRC-AIMS) |
United Kingdom |
rs-fMRI (whole brain) sex*dx |
Hurst exponent (H) in BOLD time-series as an index for synaptic excitation: inhibition (E:I) ratio |
68 autism (34:34) 67 TD (33:34) |
all participants 18–49 yr |
mean frame-wise displacement, FSIQ |
significant sex*dx interaction in VMPFC where interaction effect is driven by large TD > ASD effect in males and a small ASD > TD effect in females |
|
Olson et al., 2020 (ABIDE) |
International |
rs-fMRI (whole brain) sex*dx |
sex-related patterns of whole brain functional connectivity patterns and relation to ASD symptoms |
69 autism (34:35) 72 TD (36:36) |
all participants 7–17 yr |
root mean squared displacement |
sex*dx effects were identified between sensorimotor and higher-order supramodal networks, default mode network |
|
Floris et al., 2021 (ABIDE - discovery) (EU-AIMS LEAP, GENDAAR - replication) |
International, United States |
rs-fMRI (whole brain) sex*dx |
PCC-iFC, VMHC, ReHo, network degree centrality, fALFF |
ABIDE 444 autism (362:82) 575 TD (409:166) EU-AIMS LEAP 176 autism (133:43) 133 TD (85:48) GENDAAR 87 autism (43:44) 109 TD (56:53) |
all participants 7–18 yr |
mean frame-wise displacement |
sex*dx interaction identified in the dorsolateral occipital cortex, with reduced VMHC in autistic females compared to autistic males and TD controls, whereas TD females had higher VMHC than the other three groups; sex‐by‐diagnosis interaction was replicated in the larger of the two replication samples—EU‐AIMS LEAP |
Abbreviations: AAL, automated anatomical labelling; ABIDE, Autism Brain Imaging Data Exchange; ALFF, amplitude of low-frequency fluctuations; BOLD, blood oxygen level dependent; CEN, central executive network; CFSA, Cambridge Family Study of Autism; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; DMPFC, dorsomedial prefrontal cortex; EU-AIMS, European Autism Interventions - A Multicentre Study for Developing New Medications; fALFF, fractional amplitude of low frequency fluctuations; FSIQ, full scale intelligence quotient; GENDAAR, Gender Exploration of Neurogenetics and Development to Advance Autism Research; iFC, intrinsic functional connectivity; LEAP, Longitudinal European Autism Project; lFCD, local functional connection density; LI, laterality index; MRC-AIMS, Medical Research Council Autism Imaging Multicentre Study; MTG, middle temporal gyrus; NAcc, nucleus accumbens; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PPC, posterior parietal cortex; ReHo, regional homogeneity; ROI, region-of-interest; SFG, superior frontal gyrus; SN, salience network; STS, superior temporal sulcus; TPJ, temporo-parietal junction; TR, repetition time; VMHC, voxel-mirrored homotopic connectivity; VMPFC, ventromedial prefrontal cortex.
Table 5.
Significant findings of investigations of sex/gender-by-diagnosis interactions and sex/gender-stratified analysis of autism-control differences in brain activation and neural responses to tasks (task fMRI).
| Study | Country of origin for study sample | Method (‘sex’ or ‘gender’ is based on the term used in the study) | Metrics/Outcome measure | Sample size (M:F) | Age range | Covariates in analysis | Notable results (‘sex’ or ‘gender’ is based on the term used in the study) |
|---|---|---|---|---|---|---|---|
|
Beacher et al., 2012a |
United Kingdom |
task fMRI (whole-brain) sex*dx |
brain activation during performance of mental rotation and verbal fluency tasks |
29 autism (15:14) 32 TD (16:16) |
no range; AS 32.8 ± 9.1 yr TD 30.4 ± 7.7 yr |
realignment movement, proxy measure of intelligence (NART) |
significant sex*dx interaction across occipital, temporal, parietal, middle frontal regions (left precuneus, left middle occipital gyrus, left inferior temporal gyrus, right middle occipital gyrus) with greater activation in males with AS compared to females with AS and TD males |
|
Schneider et al., 2013 |
Germany |
task fMRI (whole-brain) gender stratified |
empathic responses and task-relevant neural activation patterns |
28 autism (15:13) 28 TD (15:13) |
all participants 18–55 yr |
realignment parameters, TAS-20 (Toronto Alexithymia Scores) |
autistic females had decreased activation in the midbrain, limbic regions (left amygdala), right PAG (ASD < TD); no significant difference in male groups |
|
Holt et al., 2014 (CFSA) |
United Kingdom |
task fMRI (whole-brain) sex*dx + sex stratified |
performance/ neural response on Eyes task |
49 autism (33:16) 40 unaffected siblings (12:28) 40 TD (20:20) |
all participants 12–18 yr |
age, verbal IQ |
no significant sex*dx interaction effects autistic females and unaffected female siblings both had decreased activation in the left dorsal anterior ACC, anterior PFC, inferior prefrontal gyrus, DLPFC, retrosubicular area – suggesting neuro-endophenotype in females; sex stratified analysis in males did not show evidence of neuro-endophenotype in male groups |
|
Kirkovski et al., 2016a |
Australia |
task fMRI (whole-brain + ROIs: medial PFC, right TPJ – including STS) sex stratified |
performance/ neural response in social under-standing task |
27 autism (13:14) 23 TD (11:12) |
all participants 19–56 yr |
motion realignment parameters (not specified), handedness |
males - right posterior superior temporal sulcus (ASD < TD); no significant difference in female groups |
|
Lai et al., 2019b (MRC-AIMS) |
United Kingdom |
task fMRI (ROIs: VMPFC, right TPJ) sex*dx |
neural response during mentalizing and self-referential cognition |
57 autism (29:28) 62 TD (33:29) |
all participants 18–45 yr |
age, FSIQ |
males – right TPJ and VMPFC (ASD < TD); no significant difference in female groups |
|
Lawrence et al., 2020b(GENDAAR) |
United States |
task fMRI (whole-brain limited to gray matter voxels and bilateral NAcc ROI) sex*dx + sex stratified |
social reward processing during instrumental implicit learning task |
82 autism (43:39) 72 TD (39:33) |
all participants 8–17 yr |
site/scanner, age, pubertal development, general cognitive ability |
ROI analyses: compared to same-sex TD counterparts, autistic males and females showed no significant differences in NAcc activity whole-brain analyses: autistic females showed greater neural activity (ASD > TD) to social rewards in lateral frontal regions (VLPFC, OFC, anterior insula, and other frontal and temporal regions); autistic males did not significantly differ from TD males no significant sex*dx interaction in NAcc ROI or whole-brain analyses |
Abbreviations: ACC, anterior cingulate cortex; AS, Asperger syndrome; CFSA, Cambridge Family Study of Autism; DLPFC, dorsolateral prefrontal cortex; FSIQ, full scale intelligence quotient; GENDAAR, Gender Exploration of Neurogenetics and Development to Advance Autism Research; MRC-AIMS, Medical Research Council Autism Imaging Multicentre Study; NART, National Adult Reading Test; NAcc, nucleus accumbens; OFC, orbitofrontal cortex; PAG, periaqueductal gray; PFC, prefrontal cortex; ROI, region-of-interest; STS, superior temporal sulcus; TPJ, temporo-parietal junction; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex.
Three studies highlighted significant autism-control differences pertaining to default mode network (DMN) connectivity when stratified by sex/gender. Ypma et al. (2016) found a significant reduction in DMN intra-connectivity in both autistic males and females compared to typically developing controls (N = 91). Kozhemiako et al. (2020) also reported DMN functional underconnectivity in both autistic females and males compared to typically developing controls (N = 390). A similar pattern of autism-control differences in DMN connectivity was observed in a methodologically rigorous study by Floris et al. (2021). In a large discovery sample, Floris and colleagues (2021) reported sex-independent diagnostic effect involving DMN underconnectivity which was robust across different preprocessing pipelines. It is important to note that findings of autism-control DMN differences from these three studies may rely on a shared neuroimaging data source (Autism Brain Imaging Data Exchange; ABIDE). Furthermore, while there is support for a sex-independent role of DMN in autism (Floris et al., 2021, Kozhemiako et al., 2020), the DMN could also involve sex-dependent features measured by other resting-state fMRI metrics. For example, Trakoshis et al. (2020) reported a sex-by-diagnosis interaction in a neural system involving DMN by examining a time-series complexity metric, the Hurst component, as an index for excitation-inhibition balance. These findings show potential sex-differential features of the DMN in autism.
The identified task fMRI studies were very heterogeneous and involved distinct cognitive tasks, which evoked neural responses involving different brain regions. Of the four task fMRI studies that specifically examined sex/gender-by-diagnosis interaction effects, different patterns of interactions were found with various cognitive tasks. Mental rotation tasks (N = 61, Beacher et al., 2012a) evoked greater activation in males with Asperger syndrome compared to typically developing males and females with Asperger syndrome and this sex-by-diagnosis interaction was significant across occipital, temporal, parietal, middle frontal regions. Neural responses to ‘Reading the Mind in the Eyes’ task (N = 89, Holt et al., 2014) did not show any significant sex-by-diagnosis interaction effects. Neural responses to mentalizing and self-referential cognition (N = 119, Lai et al., 2019b) showed hypoactive right temporo-parietal junction and ventromedial prefrontal cortex respectively in autistic males compared to typically developing males; however, no significant difference in neural responses was found in the female groups. Social reward processing during an instrumental implicit learning task (N = 154, Lawrence et al., 2020b) did not show any significant sex-by-diagnosis interaction effects; however, when examining autism-control differences stratified by sex, autistic females showed greater neural activity to social rewards in lateral frontal regions than typically developing females, yet no differences in neural activity were found between the male groups.
4.5.4. Structural MRI
Lastly, there were 15 structural MRI studies that reported significant sex/gender-by-diagnosis interaction effects and/or autism-control differences from sex/gender-stratified analysis (Table 6). Two studies (N = 152, Retico et al., 2016; N = 85, Schumann et al., 2010) conducting sex/gender-stratified analysis with overlapping age range of toddlers and preschoolers, reported similar autism-control differences where both autistic males and females featured greater gray matter volumes in the frontal and temporal regions compared to typically developing individuals. There was also an overlap of findings where two studies (N = 196, Ecker, 2019; N = 193, Irimia et al., 2018) reported significant interaction effects in the area of the parahippocampal cortex. There was one study (N = 654, Williams et al., 2020) that replicated the significant group-by-linear age-by-sex interaction in hippocampal volumes that was previously reported (N = 859, Zhang et al., 2018); however, other interaction effects that were found in caudate and putamen volumes were not replicated. The largest study (N = 3,607, Postema et al., 2019) included in this systematic review examined structural brain asymmetry and a significant sex-by-diagnosis interaction was found in the asymmetry index for cortical thickness in the rostral anterior cingulate. When analyzed within male and female groups separately, this asymmetry index was associated with a diagnosis effect in males but not females. Finally, a study took a unique approach to examine overall/global pattern of sex modulation instead of the localization of sex/gender-by-diagnosis effects across cortical thickness, surface area, volume, mean absolute curvature, and subcortical volume (N = 839, Hammill et al., 2021) and found that the overall spatial involvement of atypical neuroanatomy in autistic females and males differed qualitatively in cortical curvature and subcortical volume.
Table 6.
Significant findings of investigations of sex/gender-by-diagnosis interactions and sex/gender-stratified analysis of autism-control differences in brain morphometry (structural MRI).
| Study | Country of origin for study sample | Method (‘sex’ or ‘gender’ is based on the term used in the study) |
Metrics/Outcome measure | Sample size (M:F) | Age range | Covariates in analysis | Notable results (‘sex’ or ‘gender’ is based on the term used in the study) |
|---|---|---|---|---|---|---|---|
|
Schumann et al., 2010 (longitudinal study) |
United States |
sMRI (ROIs: frontal gray, temporal gray, parietal gray, occipital gray, cingulate gray, total gray, total white, total cerebral volume) gender stratified |
cerebral GM and WM volume |
41 autism (32:9) 44 TD (32:12) |
all participants 1–2 yr at start of study final visit: autism 1.8–5.6 yr (M) 2.2–4.8 yr (F) TD 1.0–5.3 yr (M) 1.0–5.1 yr (F) |
age at scan |
autistic females showed more pronounced abnormal growth profile in more brain regions than autistic males males – frontal and temporal GM volumes (ASD > TD); females – total cerebrum, WM, GM, frontal and temporal volumes (ASD > TD) |
|
Lai et al., 2013b (MRC- AIMS) |
United Kingdom |
sMRI (whole-brain) sex*dx |
brain GM and WM volume |
60 autism (30:30) 60 TD (30:30) |
all participants 18–49 yr |
age |
significant sex*dx interaction in two clusters in bilateral temporo-parieto-occipital regions, involving posterior portion of bilateral cingulum, ILF, CC (splenium), right AF with the females show ASD > TD and males show ASD = TD significant sex*dx interaction in two clusters involving internal capsule bilaterally at the level around the basal ganglia and thalamus where the females show ASD < TD and males show ASD > TD |
|
Schaer et al., 2015 (ABIDE) |
Inter-national |
sMRI (whole-brain) sex*dx |
local cortical morphometry (volume, thickness, gyrification) |
106 autism (53:53) 104 TD (53:51) |
all participants 6–56 yr |
site, age, cortical volume |
local cortical volume: no sex*dx interaction local cortical thickness: no sex*dx interaction local cortical gyrification: significant sex*dx interaction in VMPFC/OFC cluster |
|
Sussman et al., 2015 (POND) |
Canada |
sMRI (ROIs: cortical segmentation into 78 brain regions; volumes for cerebellum, hippocampus, striatum, pallidum, thalamus and associated sub-regions (MAGeT Brain algorithm) sex*dx |
total brain volume, total surface area, mean cortical thickness |
72 autism (61:11) 138 TD (116:22) |
all participants 4–18 yr |
age |
no sex*dx interaction effect was found for total surface area or mean cortical thickness significant sex*dx interaction was found in total brain volume, relative volume of cerebellar lobules 8b and 10, total hippocampus, left hippocampus and hippocampal subiculum |
|
Retico et al., 2016 |
Italy |
sMRI (whole-brain) gender stratified |
GM and WM volume, CSF volume, TIV (sum of GM, WM, CSF volumes) |
76 autism (38:38) 76 TD (38:38) |
autism 2.1–7.3 yr TD 1.8–7.4 yr |
none reported |
autistic males showed increased GM volume in left middle occipital gyrus and right superior temporal gyrus compared to TD males autistic females showed increased GM volume in bilateral frontal regions, right anterior cingulate cortex, right cerebellum compared to TD females |
|
Irimia et al., 2018 (GENDAAR) |
United States |
sMRI (ROIs: 165 brain regions identified using a probabilistic atlas to parcel a total of 74 cortical structures [gyri and sulci] in each hemisphere and the brain stem) sex*dx |
GM thickness, volume, cortical area, mean curvature, CD |
110 autism (55:55) 83 TD (43:40) |
no range; autism 12.7 ± 2.8 yr TD 13.0 ± 3.0 yr |
age, site |
significant sex*dx interaction in temporal pole, parahippocampal gyrus, superior temporal gyrus, occipital poles, cuneus |
|
Zhang et al., 2018 (ABIDE) |
Inter-national |
sMRI (whole-brain) sex*dx age*sex*dx |
GM and WM volume and subcortical structure volumes |
401 autism (351:50) 458 TD (378:80) |
all participants 6.5–64.0 yr |
FSIQ, total brain volume |
no significant sex*dx interaction; age*sex*dx interaction in total GM, total WM, hippocampal volumes, caudate volumes and putamen volumes |
|
Ecker, 2019 (MRC-AIMS) |
United Kingdom |
sMRI (whole-brain) sex*dx |
cortical thickness |
98 autism (49:49) 98 TD (51:47) |
autism 18–41 yr TD 18–42 yr |
total GM volume |
significant interaction in bilateral parahippocampal and entorhinal cortex, fusiform and lingual gyrus, inferior or middle temporal lobe |
|
Bosco et al., 2019 |
Italy |
sMRI (ROIs: brainstem) gender stratified |
volume and shape of brainstem |
76 autism (38:38) 76 TD (38:38) |
autism 2.1–7.3 yr TD 1.8–7.4 yr |
age, total intracranial volume |
brainstem volume in males (ASD > TD); no significant autism-control difference in female groups |
|
Peterson et al., 2019 |
United States |
sMRI and Arterial Spin Labeling (ASL) (whole-brain) sex*dx |
regional cerebral blood flow (rCBF) |
44 autism (32:12) 66 TD (50:16) |
autism 5.9–60.7 yr TD 6.9–59.0 yr |
age, FSIQ, psychotropic medication use |
significant sex*dx effect on rCBF in limbic regions (subgenual ACC, ventral striatum, amygdala, parietal WM) |
|
Postema et al., 2019 (ENIGMA) |
Inter-national |
sMRI (whole-brain) sex*dx + sex stratified |
structural brain asymmetry for multiple brain regional and global hemispheric measures (i.e., cortical thickness, cortical surface area, subcortical volume) |
1778 autism (1504:274) 1829 TD (1400:429) |
all participants 2–64 yr |
corrected for ‘data set’ as a random effect in analysis (to account for heterogeneity of imaging protocols) |
significant sex*dx interaction in the rostral anterior cingulate thickness asymmetry index (AI); this AI had shown a significant effect of diagnosis in the primary analysis. In analysis within the sexes separately, this AI was associated with diagnosis in males but not females |
|
Bedford et al., 2020 (MRC-AIMS, CFSA, ABIDE, Hospital for Sick Children, NIMH) |
Inter-national |
sMRI (whole-brain) sex*dx + sex stratified |
cortical morphometry (cortical thickness, surface area, cortical volume, total GM, total WM, total brain volume) |
491 autism (362:129) 836 TD (481:355) |
all participants 2–65 yr |
age (and using a prospective meta-analytic technique to account for inter-site differences) |
no significant sex*dx interactions found autistic males had significantly greater cortical volume, mean cortical thickness in the bilateral superior temporal, inferior frontal, and right precentral gyri compared to TD males; WM volume was also greater in autistic males compared to TD males; no differences in total surface area or GM volume autistic females had greater mean cortical thickness in the bilateral prefrontal and occipital cortices, and left posterior parietal cortex and pre- and postcentral gyri compared to TD females; no differences observed for total brain volume, total surface area, cortical volume, GM or WM |
|
Williams et al., 2020 (ABIDE) |
Inter-national |
sMRI (whole-brain) sex*dx*linear age (replication of Zhang et al., 2018) |
subcortical allometric and volumetric group differences |
302 autism (265:37) 352 TD (283:69) |
autism 7.0–26.9 yr TD 6.5–26.9 yr |
FSIQ, total brain volume |
replicated significant sex*dx*linear age interaction in hippocampal volumes found by Zhang et al. (2018) |
|
Olafson et al., 2021 (MRC-AIMS, CFSA, ABIDE, Hospital for Sick Children, etc.) |
Inter-national |
sMRI (whole-brain) sex stratified |
Boundary sharpness coefficient (BSC) – proxy for alterations in micro-structure at cortical GWM boundary |
415 autism (303:112) 721 TD (438:283) |
all participants 2–65 yr |
age, FIQ (and using a prospective meta-analytic technique to account for inter-site differences) |
females with ASD showed significantly greater BSC in bilateral superior parietal gyrus and superior temporal gyrus males with ASD showed significantly greater BSC in bilateral inferior temporal gyrus and left inferior frontal lobe |
|
Hammill et al., 2021 (POND, Hospital for Sick Children) |
Canada |
sMRI (whole-brain) overall sex modulation pattern (local magnitude model – quantitative sex modulation; spatial dissimilarity model – qualitative sex modulation) |
cortical thickness, surface area, volume, mean absolute curvature, and subcortical volume |
373 autism (299:74) 466 TD (240:226) |
all participants 2.8–50 yr |
total brain volume (and its exponential transform), age (linear or quadratic), their interactions with sex, and scanner version – determined via model selection |
no evidence supporting quantitative sex modulation; some evidence supporting qualitative sex modulation in terms of cortical mean absolute curvature and subcortical volume |
Abbreviations: ABIDE, Autism Brain Imaging Data Exchange; ACC, anterior cingulate cortex; CC, corpus callosum; CD, connectivity density; CFSA, Cambridge Family Study of Autism; CR, corona radiata; CSF, cerebrospinal fluid; ENIGMA, Enhancing Neuro Imaging Genetics through Meta-Analysis; FSIQ, full scale intelligence quotient; GM, gray matter; GWM, gray-white matter; MRC-AIMS, Medical Research Council Autism Imaging Multicentre Study; NIMH, National Institute of Mental Health; OFC, orbitofrontal cortex; PFC, prefrontal cortex; POND, Province of Ontario Neurodevelopmental Disorders Network; ROI, region-of-interest; TIV, total intracranial volume; WM, white matter
4.6. Evaluation of reported effect size against minimally detectable effect (MDE)
A sensitivity power analysis was conducted for ten studies that were identified to have the largest total sample size and/or largest autistic female group for each neuroimaging modality (Table 7), as these studies represent the datasets with the largest power so far in detecting significant sex/gender-modulation findings for each imaging modality. Where the information was available, reported effect sizes were compared with the MDE, computed based on α = 0.05 and power = 0.8. Of the six among ten studies that reported significant sex/gender-modulating effects, four studies may have sufficient power to detect the reported sizes of effects (i.e., reported effect size > MDE). These findings include: 1) sex-by-diagnosis interaction effects in FA of the anterior segment of the arcuate fasciculus bilaterally, uncinate fasciculus bilaterally; autism-control differences in the left uncinate fasciculus that was significant in males only (DTI, N = 213, Zeestraten et al., 2017); 2) reduced longitudinal functional connectivity density (lFCD) in the anterior thalamus in autistic males compared to typically developing males (rs-fMRI, N = 1,491, Tomasi & Volkow, 2019); 3) sex-by-diagnosis interaction effects in rostral anterior cingulate thickness asymmetry index; autism-control differences in this same asymmetry index that was significant in males only (sMRI, N = 3,607, Postema et al., 2019); and 4) autism-control differences in neural activity to social rewards in lateral frontal regions that was significant in females only (task fMRI, N = 154, Lawrence et al., 2020b), although the reported effect sizes here were based on extracted parameter estimates from cluster-based inference, hence were likely exaggerated (i.e., suffering from the ‘double dipping’ problem (Kriegeskorte et al., 2009)). In Table 7, there were studies that reported significant sex/gender-by-diagnosis interaction effects and/or autism-control differences where some of the reported effect sizes were smaller than the estimated MDE, which suggests increased possibility that some of these tests may be underpowered and may have resulted in false-positive findings.
Table 7.
Comparison of minimally detectable effect (MDE) and reported effect sizes of the largest studies (Ntotal and Nautism(f)) by neuroimaging modality.
| Imaging Modality (Ntotal and/or Nautism(f)) | Study | Method (‘sex’ or ‘gender’ is based on the term used in the study) | Metrics/Outcome measure | Sample size (M:F) | Minimally detectable effect size (MDE; Cohen’s d)aα = 0.05 power = 0.8 | Reported effect size (Cohen’s d) where P-values are significant at a level <0.05 |
||
|---|---|---|---|---|---|---|---|---|
| sex/gender*dx NOTE: F converted to Cohen’s d (Lenhard and Lenhard, 2016) | sex/gender-stratified (M) | sex/gender-stratified (F) | ||||||
| DTIb Ntotal = 213 |
Zeestraten et al., 2017 (MRC-AIMS) United Kingdom |
ROI sex*dx + sex stratified |
FA | 98 autism (61:37) 115 TD (61:54) |
sex*dx (0.39) sex-stratified (males, 0.51) (females, 0.60) |
Anterior segment of AF left (0.41); right (0.46) Long segment of AF left (0.32) CING left (0.38); right (0.33) Uncinate left (0.43); right (0.49) IFOF left (0.34); right (0.34) |
Anterior segment of AF left (0.49); right (0.41) Long segment of AF left (0.37) CING left (0.50) Right (0.43) Uncinate left (0.52); right (0.48) IFOF left (0.47); right (0.45) Posterior segment of AF left (0.31); right (0.29) ILF left (0.41); right (0.34) |
No significant diagnostic effect in females |
| DTIb Nautism(f) = 55 |
Irimia et al., 2017 (GENDAAR) | whole-brain sex*dx | GM thickness, volume, cortical area, mean curvature, CD | 110 autism (55:55) 83 TD (43:40) |
sex*dx (0.41) | CD (0.19) | N/A | N/A |
| MEG Ntotal = 75 Nautism(f) = 8 |
Yoshimura et al., 2021 | (whole-brain) gender* dx |
bilateral auditory cortical response (P1m) | 29 autism (21:8) 46 TD (41:5) |
gender*dx (0.66) | No significant gender*dx interaction found | N/A | N/A |
| MRS Ntotal = 174 Nautism(f)=15 |
O’Neill et al., 2020 | Near whole-brain sex*dx |
metabolite concentration (N-acetyl compounds, glutamate + glutamine, creatine + phosphor-creatine, choline compounds) | 78 autism (63:15) 96 TD (69:27) |
sex*dx (0.43) | No effect size reported (only reported p = 0.001) | N/A | N/A |
| PET Ntotal = 121 Nautism(f) = 4 |
Mitelman et al., 2018 | ROI group* sex 3 × 2 ANOVA |
GM and WM metabolic rates | 25 autism (21:4) 41 schizo-phrenia (32:9) 55 TD (29:26) |
group*sex (0.57)a | no significant group*sex interaction; d = 0.068; p = 0.79 | N/A | N/A |
| rs-EEG Ntotal = 46 Nautism(f) = 3 | Saunders et al., 2016 | whole-brain group* gender 4 × 2 ANOVA |
128-channel EEG oscillation coherence |
13 autism (10:3) 10 anxiety (2:8) 11 ADHD (7:4) 12 TD (7:5) |
group*gender (1.02)a | Interaction between gender and experimental group for interhemispheric coherence scores that was approaching significance: Alpha eyes closed frontal-frontal d = 0.231; p = 0.053 Alpha eyes open central-central d = 0.219; p = 0.072 Theta eyes open central-central d = 0.218; p = 0.074 |
N/A | N/A |
|
rs-fMRI Ntotal =1491 Nautism(f) =91 |
Tomasi and Volkow, 2019 (ABIDE) |
whole-brain sex stratified |
lFCD, LI, ALFF, seed-voxel correlation maps |
656 autism (565:91) 835 TD (602:233) |
sex-stratified (males, 0.16) (females, 0.35) |
N/A |
lFCD in anterior thalamus (ASD < TD) (PFWE < 0.005, effect size: 0.1807 < d < 0.3034, df = 1162) |
none reported; no significant group differences in thalamic ln(IFCD) between autistic and TD females (P = 0.55) |
| sMRI Ntotal =3607 Nautism(f) =274 |
Postema et al., 2019 (ENIGMA) |
whole-brain sex*dx + sex stratified |
structural brain asymmetry for multiple brain regional and global hemispheric measures (i.e., cortical thickness, cortical surface area, subcortical volume) |
1778 autism (1504:274) 1829 TD (1400:429) |
sex*dx (0.09) sex-stratified (males, 0.10) (females, 0.22) |
rostral anterior cingulate thickness asymmetry index (d = 0.11) |
rostral anterior cingulate thickness asymmetry index (d = -0.17, P = 1.4 × 10–5) |
no group differences in rostral anterior cingulate thickness asymmetry index (d = 0.11, P = 0.165) |
|
task fMRI Ntotal =154 Nautism(f) =39 |
Lawrence et al., 2020b (GENDAAR) |
whole-brain and ROI sex*dx + sex stratified |
social reward processing during instrumental implicit learning task |
82 autism (43:39) 72 TD (39:33) |
sex*dx (0.45) sex-stratified (males, 0.63) (females, 0.67) |
ROI analyses: no sex*dx interaction found Whole-brain analysis: no sex*dx interaction found |
ROI analyses: no differences found between autism and control groups when stratified by sex Whole-brain analysis: no differences found between autism and control groups when stratified by sex |
ROI analyses: no differences found between autism and control groups when stratified by sex Whole-brain analysis: Left frontal/insular cluster (d = 0.87) Right insular/temporal cluster (d = 0.89) Left frontal cluster (d = 0.80) |
| TMS-EEG Ntotal =42 Nautism(f) =12 |
Kirkovski et al., 2016b | ROI sex stratified |
cortical function and connectivity | 22 autism (10:12) 20 TD (11:9) |
sex stratified (males, 1.29) (females, 1.30) |
N/A | no differences found between autism and control groups when stratified by sex | no differences found between autism and control groups when stratified by sex |
Abbreviations: ABIDE, Autism Brain Imaging Data Exchange; AF, arcuate fasciculus; ALFF, amplitude of low-frequency fluctuations; CD, connectivity density; CING, cingulum; CSF, cerebrospinal fluid; ENIGMA, Enhancing Neuro Imaging Genetics through Meta-Analysis; GABA, gamma-aminobutyric acid; GENDAAR, Gender Exploration of Neurogenetics and Development to Advance Autism Research; GM, gray matter; IFOF, interior frontal occipital fasciculus; ILF, inferior longitudinal fasciculus; lFCD, local functional connection density; LI, laterality index; MRC-AIMS, Medical Research Council Autism Imaging Multicentre Study; PFC, prefrontal cortex; ROI, region-of-interest; TPJ, temporo-parietal junction; WM, white matter.
Due to limitations of the R pwr package for MDE calculations for 3 × 2 and 4 × 2 ANOVA designs, the MDE was calculated assuming equal sample sizes using WebPower (Zhang & Yuan, 2018). This value is expected to be smaller than the MDE for a study with unequal sample sizes.
DTI study conducted by Zeestraten et al., (2017) had the largest total sample size but did not have the largest autistic female sample. A second DTI study (Irimia et al., 2017) which had the largest autistic female sample size was therefore also included.
5. Discussion
5.1. Overview of autism neuroscience research
This systematic review demonstrates a significant gap in the literature on the modulating effects of sex or gender in brain structure and function associated with autism. Screening over 10,000 articles generated an overview of the current research landscape (Fig. 4) and within the ‘Brain Structure & Function’ category (N = 1,428), it was notable that there was a 434:4 discrepancy in the number of male-only versus female-only studies. The search yielded 851 neuroimaging studies; however, only 69 of these have formally evaluated sex/gender-by-diagnosis interaction effects and/or conducted sex/gender-stratified analyses. This is a reflection of the current autism neuroscience literature that largely ignores or fails to formally examine sex and gender variables. Included studies were very heterogeneous in their design, sample characteristics, and brain metrics analyzed. So far, there is no consensus for specific regions or neural networks that consistently show sex/gender-modulating effects in autism.
5.2. Studies reporting significant versus non-significant findings
A quantitative evaluation of study features showed that total sample size was the most important feature associated with whether any given study reported significant findings; however, the influence of this variable on the results was not linear. In the literature to date, with sample sizes greater than 83 but fewer than 265, there was a higher likelihood for a study to report a significant finding; when the total sample size was greater than or equal to 265, the studies were less likely to report a significant finding. It is important to note that of the 69 neuroimaging studies, 20 studies had a total sample size greater than 265 where 80% (16/20 studies) were multi-site studies – thus, there is an increased likelihood of noise introduced from variability between sites (e.g., differences in scanners, data acquisition protocols, and quality control measures). These observed patterns should be interpreted with caution as our findings are simply a reflection of the characteristics of available neuroimaging studies so far (and not meant to provide a deterministic guide of the ideal ‘threshold’ for study sample sizes). Evaluation of the MDE of ten studies with the largest total sample size and/or largest autistic female group demonstrated that significant effects observed with large sample sizes tend to be quite modest in the strength of the effect, suggesting the need for caution in interpreting the biological implications. So far four studies reported significant results with univariate analyses of specific brain regions/white matter tracts of interest; however, some reported effect sizes were smaller than the estimated MDE, which suggests an increased likelihood that some of these tests were not sufficiently powered and the observed effects may potentially be false positives (Marek et al., 2020). All these findings highlight that autism-control differences, even in the context of considering sex/gender-stratification, and sex/gender-modulation effects, seem small to moderate at a group-average level, reflecting the substantial heterogeneity within autism even after accounting for sex/gender-based heterogeneity (Lombardo et al., 2019). Further, published studies, when reporting significant results, may be vulnerable to finding false positives. Future investigations of sex/gender-modulation in autism neurobiology should strive to include larger total sample sizes and achieve a male-to-female ratio closer to population prevalence ratio (∼3:1) or smaller, for the analyses to be better powered while ensuring the necessary measures are considered to minimize the effects of noisy data from multi-site studies (e.g., using a harmonized protocol).
Even with sufficiently increased power (from larger sample sizes), the reported localized sex/gender-modulating effects are limited (Postema et al., 2019, Zeestraten et al., 2017), indicating that both sex/gender-independent and sex/gender-dependent localized brain characteristics should be considered to understand autism neurobiology (Floris et al., 2021). However, all these findings need to be interpreted in light of sampling/ascertainment bias and the limited external validity as shown in our risk of bias assessment. On the one hand, given the potential ‘male lens’ in the clinical recognition of autism (Fig. 1), autistic females included in these neuroimaging studies may be more phenotypically similar to males and/or with heightened autistic features (Ratto et al., 2018), therefore more likely to show brain characteristics that are similar to males (Beacher et al., 2012b). On the other hand, the included autistic females may have more co-occurring physical or psychiatric conditions (Kassee et al., 2020, Lai et al., 2019a), and hence the observed or un-observed sex/gender-modulation effects in these neuroimaging studies may be confounded by residual effects of co-occurring conditions and medication exposure that can impact brain development (Chugani, 2005).
5.3. Implications of disordinal (cross-over) sex/gender-by-diagnosis interactions
The studies that do report localized significant sex/gender-by-diagnosis interactions are mostly finding disordinal (cross-over), instead of ordinal (same-direction of effect) interactions (Widaman et al., 2012). Disordinal interactions imply a switch or reversal of the effect of one independent variable (e.g., diagnostic group: autism vs. controls), at a level of a second independent variable (e.g., sex/gender: male vs. female) where the autism-control differences for males and females are of different directions; ordinal interactions, on the other hand, imply that the effect (e.g., diagnosis) in one condition (e.g., females) is of the same direction but stronger than that in the other condition (e.g., males). For example, two resting-state fMRI studies (Alaerts et al., 2016, Smith et al., 2019) converged to show patterns of underconnectivity in autistic males and overconnectivity in autistic females, which suggest that neural connectivity atypicalities related to autism may present differently in males and females. Retico et al. (2016) have also reported male and female autistic toddlers showing increased gray matter volumes in different regions of the brain. These localized sex/gender differences correspond to a converging profile identified by a study demonstrating overall/global patterns of qualitative sex/gender differences in neuroanatomy (Hammill et al., 2021), highlighting the importance of considering how sex and gender moderate the overall autism neurobiology. It is important to note that disordinal interactions may be over-represented in the literature and reflect a reporting bias in the field, as they require less power than ordinal interactions to detect and whole-brain ANOVAs may be biased towards detecting disordinal interactions (Chavez & Wagner, 2017). Thus, disordinal effects only reflect parts of the whole picture of sex/gender-modulation in autism neuroimaging findings.
5.4. Age and sex/gender-modulation of autism neurobiology
For diffusion imaging, four studies showed significant findings in the inferior frontal occipital fasciculus; however, the direction of findings diverged between studies. In comparison to same-sex typically developing controls, greater FA was reported in teenage autistic males (Bode et al., 2011) while lower FA was reported in adult autistic males (Zeestraten et al., 2017) and lower FA was reported in female autistic children and adolescents (Lei et al., 2019) and greater FA was reported in both preschool-aged autistic males and females (Andrews et al., 2019). Notably, these studies examined participants with different ages, which may be a key factor for the inconsistent findings. In typical development, FA increases with age until adulthood and then gradually declines (Lebel et al., 2010). Previous studies have found age-related differences in white matter microstructure between autistic males and typically developing males, including atypical microstructure in the thalamus and the posterior limb of the internal capsule during childhood that appeared to approach the trajectory of typically developing individuals in adolescence and adulthood (McLaughlin et al., 2018). Examination of the developmental trajectory of the corpus callosum using a cohort sequential design over nine years in autistic males from 3 to 41 years has shown atypical brain maturation in terms of FA in the anterior corpus callosum that was present only in the youngest participants (<10 years) in the autism group when stratified by age (Travers et al., 2015). Both of these longitudinal investigations consist of only male cohorts. Therefore, it is unclear whether the trajectory of white matter microstructural maturation for autistic females follows a similar or different pattern. There is a great need to consider the effects of age and developmental stage when investigating potential sex- and gender-modulating effects in autism neurobiology, especially since sex-differential brain development is known to be influenced by both organizational and activational effects, the latter continuing throughout the lifespan (Bale & Epperson, 2015), and age-related gendered contextual factors can further shape brain growth (Rippon, 2019). Investigations of diagnosis-by-age-by-sex/gender interactions with large and well-powered cross-sectional and, more ideally, longitudinal designs in the future will provide further insight.
5.5. A lack of research examining the effects of gender
This systematic review demonstrates a lack of a clear definition of the terms, ‘sex’ and ‘gender’, in over 70% of the included neuroimaging studies (N = 69) examining sex/gender-modulation effects and/or conducting sex/gender-stratified analyses. Without clear indications or measurements for sex and gender respectively, it is difficult to determine whether the observed effects are associated with sex or gender, or both. There are no studies to date that measured sex and gender variables and their effects separately. This lack of research examining the effects of gender represents a substantial knowledge gap and a missed opportunity to capture the broader variances associated with the multiple sex- and gender-related factors compared with a single, binarized sex/gender label (Joel & McCarthy, 2017). Apart from the potential sex-modulating effects that contribute to the manifestation of autism in males and females, socially constructed roles and activities can affect one’s behaviour and brain development, especially with the consideration that gender socialization begins at birth (Lai et al., 2015). Parent-child and peer relationships serve a key role in brain development and neuroplasticity; child play, for example, is a powerful peer relationship that may influence prefrontal development (Kolb & Gibb, 2011) and is often associated with gendered play patterns. Further, studies have suggested that there is a higher prevalence of gender diversity and gender dysphoria among autistic individuals compared to typically developing individuals (Dewinter et al., 2017, Hisle-Gorman et al., 2019). Affiliation to gender constructs in line with sex assigned at birth also seems lower in autistic individuals, especially in birth-assigned females (Cooper et al., 2018), which may be associated with their social experiences with expectations to conform with societal gendered norms. These experiences can influence brain development and plasticity, suggesting that gender can have potentially modulating effects on autism neurobiology over and above those of biological sex. Future investigations should formally examine the effects of gender and sex respectively (Strang et al., 2020) and explore how the relations between gender constructs and social behaviour influence brain development, structure, and function associated with autism.
5.6. Limitations of this systematic review and of the current literature
It is important to note the significant heterogeneity of the 69 neuroimaging studies included in this systematic review, with a very wide range of imaging modalities, brain metrics, and sample demographics. This makes it difficult to directly compare findings across studies. Our comparison of studies that reported significant findings with those that reported non-significant findings was limited to comparing study features where the information was available across reports, which was not exhaustive. For example, although the age range of participants would have been an important study feature to consider, there were studies that did not report this information. Therefore, our discovery pertaining to study characteristics in association with the significance of reported findings must be considered exploratory, descriptively reflecting the current literature (which is still evolving) and limited by the inconsistency of reporting and diversity of study methodologies.
Our search strategy used subheadings and keywords for broad terms, including ‘brain’ instead of more specific terms (i.e., ‘neuroimaging’), to capture all relevant studies examining the underpinnings of the autistic brains, which were then categorized by research subjects. Search queries in databases are generally limited to searching titles and abstracts and research articles tend to focus on positive findings in their abstract, which may cause bias in systematic reviews (Duyx et al., 2019). To address this potential ‘abstract reporting bias’ (Duyx et al., 2019), subheadings and keywords for (‘sex’ OR ‘gender’) were used instead of searching directly for (‘sex difference’ OR ‘gender difference’). This broader search was an attempt to capture studies that may have conducted post-hoc tests involving sex/gender, where the investigation of sex/gender differences may not have been the primary goal, and the terms ‘sex difference’ or ‘gender difference’ may not be in the title or abstract of these articles. As such, our choice of search criteria was limited to capturing articles where there was mention of ‘sex’ or ‘gender’ in the title or abstract, but not restricted to capturing only those that specifically reported sex or gender differences. Articles that examined sex/gender-modulating effects with positive and negative findings were identified at the level of full-text screening with a three-tier exclusion process (Fig. 2). The use of broader search terms and a three-tier exclusion process are notable features of this systematic review that provided a thorough and unbiased search of the available literature.
Publication bias is a widely recognized limitation for systematic reviews, where it is the ‘tendency to publish only results that are statistically or clinically significant’ (Hedin et al., 2016). The included neuroimaging studies that did report significant sex/gender-by-diagnosis interactions mainly found disordinal interactions, which are commonly reported in the literature and require less power to detect than ordinal interactions (Chavez & Wagner, 2017), which further contributes to publication bias. This systematic review provided a unique approach of comparing the MDE and reported effect sizes for a quantitative examination and synthesis of the literature. The comparison of the MDE and reported effect sizes showed that some of the reported statistically significant sex/gender-modulating effects may potentially be false positive findings – this may be a sign that published studies are prone to reporting positive findings.
In addition, studies that reported significant findings were unlikely to be all independent since there were a number of studies utilizing common open sources of neuroimaging data (e.g., the Autism Brain Imaging Data Exchange; ABIDE) and it is unclear the extent of overlap in the datasets used. Therefore, seemingly replicated findings of (partially) similar patterns of sex/gender-modulation (e.g., functional overconnectivity in autistic females vs. underconnectivity in autistic males (Alaerts et al., 2016, Kozhemiako et al., 2020, Tomasi and Volkow, 2019)) or a lack thereof (e.g., sex/gender-independent DMN functional underconnectivity (Floris et al., 2021, Kozhemiako et al., 2020)) should be interpreted with caution, especially considering the insufficient external validity in making inferences to the wider autism population. Moving forward, efforts to examine the reproducibility of findings across independent samples (Floris et al., 2021) and to identify critical sources of heterogeneity (King et al., 2019, Tang et al., 2020) will remain the primary foci of future neuroimaging studies of autism.
5.7. Conclusion
This systematic review highlights a significant research gap in understanding sex and gender effects in the human autistic brains. The available studies that attempted to investigate these effects thus far have yet to identify converging regions or networks with consistent sex/gender-modulating effects. Despite at least three well-powered studies identifying specific patterns of significant sex/gender-modulation of autism-control differences, many other studies might not have sufficient statistical power to detect significant sex/gender-by-diagnosis interaction effects or might be at risk of reporting false-positive findings. Future investigation of sex- and gender-based heterogeneity in autism will need to use much larger, and more sex- and gender-balanced and inclusive samples to determine sex/gender-dependent and sex/gender-independent neurodevelopmental features in the autistic brains, and how they may be linked to behavioural phenotypes in autistic individuals of different sexes and genders. Effects associated with constructs of gender should be formally measured and investigated, alongside those of sex, to address this significant research gap.
CRediT authorship contribution statement
Kelly Mo: Conceptualization, Methodology, Formal analysis, Visualization, Writing – original draft, Writing - review & editing. Tara Sadoway: Validation, Writing - review & editing. Sarah Bonato: Methodology, Writing - review & editing. Stephanie H. Ameis: Writing - review & editing. Evdokia Anagnostou: Writing - review & editing. Jason P. Lerch: Writing - review & editing. Margot J. Taylor: Writing - review & editing. Meng-Chuan Lai: Supervision, Funding acquisition, Conceptualization, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Caroline Kassee for support and guidance with initial planning and organization of the systematic review, Harrison Duong for assistance with the review of the literature, and Mark Palmert and Peter Szatmari for valuable discussions. KM is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship-Master’s (CGS M) from the Canadian Institutes of Health Research (CIHR). SHA currently receives funding from the National Institute of Mental Health (R01MH114879), CIHR, the Academic Scholars Award from the Department of Psychiatry, University of Toronto, and CAMH Foundation. EA is supported by funding from CIHR, National Institute of Health, US Department of Defense, Ontario Brain Institute, Brain Canada, Genome Canada, Ontario Research Fund, Azrieli Foundation, Autism Speaks, HRSA, Canada Research Chairs Program, and Dr. Sims Chair in Autism. M-CL is supported by the Academic Scholars Award from the Department of Psychiatry, University of Toronto, the Ontario Brain Institute via the Province of Ontario Neurodevelopmental Disorders (POND) Network (IDS-I l-02), the CIHR (PJT 159578 and a CIHR Sex and Gender Science Chair, GSB 171373), the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario, and the Slaight Family Child and Youth Mental Health Innovation Fund via the CAMH Foundation.
EA has received consultation fees from Roche, in kind support from AMO Pharma, and editorial honorarium from Wiley.
Footnotes
Note: the definitions of sex and gender was adopted from the World Health Organization (2014) where ‘sex’ refers to ‘the biological and physiological characteristics that define men and women,’ and ‘gender’ refers to ‘the socially constructed roles, behaviours, activities, and attributes that a given society considers appropriate for men and women.’
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102811.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alaerts K., Swinnen S.P., Wenderoth N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cognit. Affect. Neurosci. 2016;11(6):1002–1016. doi: 10.1093/scan/nsw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. fifth ed. American Psychiatric Publishing; Arlington: 2013. [Google Scholar]
- Andrews D.S., Lee J.K., Harvey D.J., Waizbard-Bartov E., Solomon M., Rogers S.J., Nordahl C.W., Amaral D.G. A longitudinal study of white matter development in relation to changes in autism severity across early childhood. Biol. Psychiatry. 2021;89(5):424–432. doi: 10.1016/j.biopsych.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D.S., Avino T.A., Gudbrandsen M., Daly E., Marquand A., Murphy C.M., Lai M.-C., Lombardo M.V., Ruigrok A.N.V., Williams S.C., Bullmore E.T., The Mrc Aims Consortium, nullSuckling J., Baron-Cohen S., Craig M.C., Murphy D.G.M., Ecker C. In Vivo Evidence of Reduced Integrity of the Gray-White Matter Boundary in Autism Spectrum Disorder. Cerebral Cortex. 2017;27(2):877–887. doi: 10.1093/cercor/bhw404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D.S., Lee J.K., Solomon M., Rogers S.J., Amaral D.G., Nordahl C.W. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J. Neurodevel. Disord. 2019;11(1):32. doi: 10.1186/s11689-019-9291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asperger H. Die „Autistischen Psychopathen” im Kindesalter. Archiv für Psychiatrie und Nervenkrankheiten. 1944;117(1):76–136. doi: 10.1007/BF01837709. [DOI] [Google Scholar]
- Beacher F.D.C.C., Radulescu E., Minati L., Baron-Cohen S., Lombardo M.V., Lai M.-C., Walker A., Howard D., Gray M.A., Harrison N.A., Critchley H.D., Tsakiris M. Sex Differences and autism: brain function during verbal fluency and mental rotation. PLoS ONE. 2012;7(6):e38355. doi: 10.1371/journal.pone.0038355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Epperson C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18(10):1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher F.D., Minati L., Baron-Cohen S., Lombardo M.V., Lai M.-C., Gray M.A., Harrison N.A., Critchley H.D. Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 2012;33(1):83–89. doi: 10.3174/ajnr.A2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford S.A., Park M.T.M., Devenyi G.A., Tullo S., Germann J., Patel R., Anagnostou E., Baron-Cohen S., Bullmore E.T., Chura L.R., Craig M.C., Ecker C., Floris D.L., Holt R.J., Lenroot R., Lerch J.P., Lombardo M.V., Murphy D.G.M., Raznahan A., Ruigrok A.N.V., Smith E., Spencer M.D., Suckling J., Taylor M.J., Thurm A., Lai M.-C., Chakravarty M.M. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol. Psychiatry. 2020;25(3):614–628. doi: 10.1038/s41380-019-0420-6. [DOI] [PubMed] [Google Scholar]
- Bletsch A., Schäfer T., Mann C., Andrews D.S., Daly E., Gudbrandsen M., Ruigrok A.N.V., Dallyn R., Romero‐Garcia R., Lai M.-C., Lombardo M.V., Craig M.C., Suckling J., Bullmore E.T., Baron‐Cohen S., Murphy D.G.M., Dell'Acqua F., Ecker C. Atypical measures of diffusion at the gray-white matter boundary in autism spectrum disorder in adulthood. Hum. Brain Mapp. 2021;42(2):467–484. doi: 10.1002/hbm.v42.210.1002/hbm.25237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, M.K., Mattila, M.-L., Kiviniemi, V., Rahko, J., Moilanen, I., Ebeling, H., Tervonen, O., & Nikkinen, J., 2011. White matter in autism spectrum disorders—Evidence of impaired fiber formation. Acta Radiologica (Stockholm, Sweden: 1987), 52(10), 1169–1174. 10.1258/ar.2011.110197. [DOI] [PubMed]
- Bosco P., Giuliano A., Delafield‐Butt J., Muratori F., Calderoni S., Retico A. Brainstem enlargement in preschool children with autism: Results from an intermethod agreement study of segmentation algorithms. Hum. Brain Mapp. 2019;40(1):7–19. doi: 10.1002/hbm.v40.110.1002/hbm.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J.D., Baron-Cohen S., Anagnostou E., Ashwin C., Betancur C., Chakrabarti B., Crawley J.N., Hoekstra R.A., Hof P.R., Lai M.-C., Lombardo M.V., Schumann C.M. Rigor in science and science reporting: Updated guidelines for submissions to Molecular Autism. Mol. Aut. 2019;10:6. doi: 10.1186/s13229-018-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez R.S., Wagner D.D. Mass univariate testing biases the detection of interaction effects in whole-brain analysis of variance. BioRxiv. 2017;130773 [Google Scholar]
- Chugani D. Pharmacological intervention in autism: targeting critical periods of brain development. Clin. Neuropsychiatry. 2005;2(6):346–353. [Google Scholar]
- Contarino V.E., Bulgheroni S., Annunziata S., Erbetta A., Riva D. Widespread focal cortical alterations in autism spectrum disorder with intellectual disability detected by threshold-free cluster enhancement. AJNR Am. J. Neuroradiol. 2016;37(9):1721–1726. doi: 10.3174/ajnr.A4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K., Smith L.G.E., Russell A.J. Gender identity in autism: sex differences in social affiliation with gender groups. J. Autism Dev. Disord. 2018;48(12):3995–4006. doi: 10.1007/s10803-018-3590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Harwood R., Kasari C. The art of camouflage: gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism Int. J. Res. Pract. 2017;21(6):678–689. doi: 10.1177/1362361316671845. [DOI] [PubMed] [Google Scholar]
- Dewinter J., De Graaf H., Begeer S. Sexual orientation, gender identity, and romantic relationships in adolescents and adults with autism spectrum disorder. J. Autism Dev. Disord. 2017;47(9):2927–2934. doi: 10.1007/s10803-017-3199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X., Biswal B.B. Similarly expanded bilateral temporal lobe volumes in female and male children with autism spectrum disorder. Biol. Psychiatry Cognit. Neurosci. Neuroimag. 2016;1(2):178–185. doi: 10.1016/j.bpsc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle-Thomas K.A.R., Card D., Soorya L.V., Wang A.T., Fan J., Anagnostou E. Metabolic mapping of deep brain structures and associations with symptomatology in autism spectrum disorders. Res. Autism Spect. Disord. 2014;8(1):44–51. doi: 10.1016/j.rasd.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvekot J., van der Ende J., Verhulst F.C., Slappendel G., van Daalen E., Maras A., Greaves-Lord K. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism Int. J. Res. Pract. 2017;21(6):646–658. doi: 10.1177/1362361316672178. [DOI] [PubMed] [Google Scholar]
- Duyx B., Swaen G.M.H., Urlings M.J.E., Bouter L.M., Zeegers M.P. The strong focus on positive results in abstracts may cause bias in systematic reviews: a case study on abstract reporting bias. Systemat. Rev. 2019;8(1):174. doi: 10.1186/s13643-019-1082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworzynski K., Ronald A., Bolton P., Happé F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(8):788–797. doi: 10.1016/j.jaac.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Ecker C. The neuroanatomy of autism spectrum disorder: an overview of structural neuroimaging findings and their translatability to the clinical setting. Autism Int. J. Res. Pract. 2017;21(1):18–28. doi: 10.1177/1362361315627136. [DOI] [PubMed] [Google Scholar]
- Ecker, C., 2019. Notice of Retraction and Replacement: Ecker et al. Association between the probability of autism spectrum disorder and normative sex-related phenotypic diversity in brain structure. JAMA Psychiatry . 2017;74(4):329-338. JAMA Psychiatry, 76(5), 549. 10.1001/jamapsychiatry.2018.4296. [DOI] [PubMed]
- Ecker C., Bookheimer S.Y., Murphy D.G.M. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet. Neurol. 2015;14(11):1121–1134. doi: 10.1016/S1474-4422(15)00050-2. [DOI] [PubMed] [Google Scholar]
- Ferri S.L., Abel T., Brodkin E.S. Sex differences in autism spectrum disorder: a review. Curr. Psychiatry Rep. 2018;20(2):9. doi: 10.1007/s11920-018-0874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris D.L., Filho J.O.A., Lai M.-C., Giavasis S., Oldehinkel M., Mennes M., Charman T., Tillmann J., Dumas G., Ecker C., Dell’Acqua F., Banaschewski T., Moessnang C., Baron-Cohen S., Durston S., Loth E., Murphy D.G.M., Buitelaar J.K., Beckmann C.F., Milham M.P., Di Martino A. Towards robust and replicable sex differences in the intrinsic brain function of autism. Mol. Autism. 2021;12:19. doi: 10.1186/s13229-021-00415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung L.K., Flores R.E., Gu M., Sun K.L., James D., Schuck R.K., Jo B., Park J.H., Lee B.C., Jung J.H., Kim S.E., Saggar M., Sacchet M.D., Warnock G., Khalighi M.M., Spielman D., Chin F.T., Hardan A.Y. Thalamic and prefrontal GABA concentrations but not GABAA receptor densities are altered in high-functioning adults with autism spectrum disorder. Mol. Psychiatry. 2021;26(5):1634–1646. doi: 10.1038/s41380-020-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A., Saviozzi I., Brambilla P., Muratori F., Retico A., Calderoni S. The effect of age, sex and clinical features on the volume of corpus callosum in pre-schoolers with autism spectrum disorder: a case-control study. Eur. J. Neurosci. 2018;47(6):568–578. doi: 10.1111/ejn.13527. [DOI] [PubMed] [Google Scholar]
- Guo X., Simas T., Lai M.-C., Lombardo M.V., Chakrabarti B., Ruigrok A.N.V., Bullmore E.T., Baron‐Cohen S., Chen H., Suckling J. Enhancement of indirect functional connections with shortest path length in the adult autistic brain. Hum. Brain Mapp. 2019;40(18):5354–5369. doi: 10.1002/hbm.v40.1810.1002/hbm.24777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammill C., Lerch J.P., Taylor M.J., Ameis S.H., Chakravarty M.M., Szatmari P., Anagnostou E., Lai M.-C. Quantitative and qualitative sex-modulations in the brain anatomy of autism. Biol. Psychiatry Cognit. Neurosci. Neuroimag. 2021 doi: 10.1016/j.bpsc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- Hedin R.J., Umberham B.A., Detweiler B.N., Kollmorgen L., Vassar M. Publication bias and nonreporting found in majority of systematic reviews and meta-analyses in anesthesiology journals. Anesth. Analg. 2016;123(4):1018–1025. doi: 10.1213/ANE.0000000000001452. [DOI] [PubMed] [Google Scholar]
- Henry T.R., Dichter G.S., Gates K. Age and gender effects on intrinsic connectivity in autism using functional integration and segregation. Biol. Psychiatry Cognit. Neurosci. Neuroimag. 2018;3(5):414–422. doi: 10.1016/j.bpsc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Hernandez L.M., Lawrence K.E., Padgaonkar N.T., Inada M., Hoekstra J.N., Lowe J.K., Eilbott J., Jack A., Aylward E., Gaab N., Van Horn J.D., Bernier R.A., McPartland J.C., Webb S.J., Pelphrey K.A., Green S.A., Geschwind D.H., Bookheimer S.Y., Dapretto M. Imaging-genetics of sex differences in ASD: Distinct effects of OXTR variants on brain connectivity. Transl. Psychiatry. 2020;10 doi: 10.1038/s41398-020-0750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisle-Gorman E., Landis C.A., Susi A., Schvey N.A., Gorman G.H., Nylund C.M., Klein D.A. Gender dysphoria in children with autism spectrum disorder. LGBT Health. 2019;6(3):95–100. doi: 10.1089/lgbt.2018.0252. [DOI] [PubMed] [Google Scholar]
- Holt R.J., Chura L.R., Lai M.-C., Suckling J., von dem Hagen E., Calder A.J., Bullmore E.T., Baron-Cohen S., Spencer M.D. “Reading the mind in the eyes”: an fMRI study of adolescents with autism and their siblings. Psychol. Med. 2014;44(15):3215–3227. doi: 10.1017/S0033291714000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., Baker P., Smith E., Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Hsu C.-T., Neufeld J., Chakrabarti B. Reduced reward-related neural response to mimicry in individuals with autism. Eur. J. Neurosci. 2018;47(6):610–618. doi: 10.1111/ejn.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J.V., Dokovna L.B., Jacokes Z.J., Torgerson C.M., Irimia A., Van Horn J.D. Resting-state functional connectivity in autism spectrum disorders: a review. Front. Psychiatry. 2016;7:205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Lei X., Torgerson C.M., Jacokes Z.J., Abe S., Van Horn J.D. Support vector machines, multidimensional scaling and magnetic resonance imaging reveal structural brain abnormalities associated with the interaction between autism spectrum disorder and sex. Front. Comput. Neurosci. 2018;12:93. doi: 10.3389/fncom.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Torgerson C.M., Jacokes Z.J., Van Horn J.D. The connectomes of males and females with autism spectrum disorder have significantly different white matter connectivity densities. Sci. Rep. 2017;7:46401. doi: 10.1038/srep46401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin K., Gotts S.J., Xu Y., Liu S., Riddell C.D., Ingeholm J.E., Kenworthy L., Wallace G.L., Braun A.R., Martin A. Overt social interaction and resting state in young adult males with autism: core and contextual neural features. Brain: A. J. Neurol. 2019;142(3):808–822. doi: 10.1093/brain/awz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D., McCarthy M., M. Incorporating sex as a biological variable in neuropsychiatric research: where are we now and where should we be? Neuropsychopharmacol. 2017;42(2):379–385. doi: 10.1038/npp.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat A.J., Shui A.M., Ghods S.S., Farmer C.A., Esler A.N., Thurm A., Georgiades S., Kanne S.M., Lord C., Kim Y.S., Bishop S.L. Sex differences in scores on standardized measures of autism symptoms: a multisite integrative data analysis. J. Child Psychol. Psychiatry. 2021;62(1):97–106. doi: 10.1111/jcpp.v62.110.1111/jcpp.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kassee C., Babinski S., Tint A., Lunsky Y., Brown H.K., Ameis S.H., Szatmari P., Lai M.-C., Einstein G. Physical health of autistic girls and women: a scoping review. Mol. Autism. 2020;11(1):84. doi: 10.1186/s13229-020-00380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.B., Prigge M.B.D., King C.K., Morgan J., Weathersby F., Fox J.C., Dean D.C., Freeman A., Villaruz J.A.M., Kane K.L., Bigler E.D., Alexander A.L., Lange N., Zielinski B., Lainhart J.E., Anderson J.S. Generalizability and reproducibility of functional connectivity in autism. Mol. Autism. 2019;10:27. doi: 10.1186/s13229-019-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkovski M., Enticott P.G., Hughes M.E., Rossell S.L., Fitzgerald P.B. Atypical neural activity in males but not females with autism spectrum disorder. J. Autism Dev. Disord. 2016;46(3):954–963. doi: 10.1007/s10803-015-2639-7. [DOI] [PubMed] [Google Scholar]
- Kirkovski M., Enticott P.G., Maller J.J., Rossell S.L., Fitzgerald P.B. Diffusion tensor imaging reveals no white matter impairments among adults with autism spectrum disorder. Psychiatry Res. 2015;233(1):64–72. doi: 10.1016/j.pscychresns.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Kirkovski M., Fuelscher I., Hyde C., Donaldson P.H., Ford T.C., Rossell S.L., Fitzgerald P.B., Enticott P.G. Fixel based analysis reveals atypical white matter micro- and macrostructure in adults with autism spectrum disorder: an investigation of the role of biological sex. Front. Integr. Neurosci. 2020;14:40. doi: 10.3389/fnint.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkovski M., Rogasch N.C., Saeki T., Fitzgibbon B.M., Enticott P.G., Fitzgerald P.B. Single pulse transcranial magnetic stimulation-electroencephalogram reveals no electrophysiological abnormality in adults with high-functioning autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 2016;26(7):606–616. doi: 10.1089/cap.2015.0181. [DOI] [PubMed] [Google Scholar]
- Kirkovski M., Suo C., Enticott P.G., Yücel M., Fitzgerald P.B. Short communication: Sex-linked differences in gamma-aminobutyric acid (GABA) are related to social functioning in autism spectrum disorder. Psychiatry Res. Neuroimaging. 2018;274:19–22. doi: 10.1016/j.pscychresns.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Kolb B., Gibb R. Brain plasticity and behaviour in the developing brain. J. Can. Acad. Child Adolesc. Psychiatry. 2011;20(4):265–276. [PMC free article] [PubMed] [Google Scholar]
- Kreiser N.L., White S.W. ASD in females: are we overstating the gender difference in diagnosis? Clin. Child. Fam. Psychol. Rev. 2014;17(1):67–84. doi: 10.1007/s10567-013-0148-9. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S.F., Baker C.I. Circular analysis in systems neuroscience: The dangers of double dipping. Nat. Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemiako, N., Nunes, A. S., Vakorin, V., Iarocci, G., Ribary, U., & Doesburg, S. M., 2020. Alterations in Local Connectivity and Their Developmental Trajectories in Autism Spectrum Disorder: Does Being Female Matter? Cerebral Cortex (New York, N.Y.: 1991), 30(9), 5166–5179. 10.1093/cercor/bhaa109. [DOI] [PubMed]
- Lai, M.-C., Ameis, S. H., & Szatmari, P. (2017). Young Women on the Autism Spectrum. In Adolescents with Autism Spectrum Disorder. Oxford University Press. https://www.oxfordclinicalpsych.com/view/10.1093/med-psych/9780190624828.001.0001/med-9780190624828-chapter-12.
- Lai M.-C., Kassee C., Besney R., Bonato S., Hull L., Mandy W., Szatmari P., Ameis S.H. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(10):819–829. doi: 10.1016/S2215-0366(19)30289-5. [DOI] [PubMed] [Google Scholar]
- Lai M.-C., Lerch J.P., Floris D.L., Ruigrok A.N.V., Pohl A., Lombardo M.V., Baron-Cohen S. Imaging sex/gender and autism in the brain: etiological implications. J. Neurosci. Res. 2017;95(1–2):380–397. doi: 10.1002/jnr.23948. [DOI] [PubMed] [Google Scholar]
- Lai M.-C., Lombardo M.V., Auyeung B., Chakrabarti B., Baron-Cohen S. Sex/gender differences and autism: setting the scene for future research. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(1):11–24. doi: 10.1016/j.jaac.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Lombardo M.V., Chakrabarti B., Baron-Cohen S., Nestler E. Subgrouping the autism “spectrum”: Reflections on DSM-5. PLoS Biol. 2013;11(4):e1001544. doi: 10.1371/journal.pbio.1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Lombardo M.V., Chakrabarti B., Ruigrok A.NV., Bullmore E.T., Suckling J., Auyeung B., Happé F., Szatmari P., Baron-Cohen S., Bailey A.J., Bolton P.F., Carrington S., Catani M., Craig M.C., Daly E.M., Deoni S.CL., Ecker C., Henty J., Jezzard P., Johnston P., Jones D.K., Madden A., Mullins D., Murphy C.M., Murphy D.GM., Pasco G., Sadek S.A., Spain D., Stewart R., Wheelwright S.J., Williams S.C. Neural self-representation in autistic women and association with “compensatory camouflaging”. Autism: Int. J. Res. Pract. 2019;23(5):1210–1223. doi: 10.1177/1362361318807159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Lombardo M.V., Suckling J., Ruigrok A.N.V., Chakrabarti B., Ecker C., Deoni S.C.L., Craig M.C., Murphy D.G.M., Bullmore E.T., Baron-Cohen S. Biological sex affects the neurobiology of autism. Brain A J. Neurol. 2013;136(9):2799–2815. doi: 10.1093/brain/awt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.-C., Szatmari P. Sex and gender impacts on the behavioural presentation and recognition of autism. Curr. Opin. Psychiatry. 2020;33(2):117–123. doi: 10.1097/YCO.0000000000000575. [DOI] [PubMed] [Google Scholar]
- Laidi C., Boisgontier J., Chakravarty M.M., Hotier S., d’Albis M.-A., Mangin J.-F., Devenyi G.A., Delorme R., Bolognani F., Czech C., Bouquet C., Toledano E., Bouvard M., Gras D., Petit J., Mishchenko M., Gaman A., Scheid I., Leboyer M., Zalla T., Houenou J. Cerebellar anatomical alterations and attention to eyes in autism. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-11883-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M., Durston S., Staal W.G., Palmen S.J.M.C., van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol. Psychiatry. 2007;62(3):262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lawrence, K. E., Hernandez, L. M., Bowman, H. C., Padgaonkar, N. T., Fuster, E., Jack, A., Aylward, E., Gaab, N., Van Horn, J. D., Bernier, R. A., Geschwind, D. H., McPartland, J. C., Nelson, C. A., Webb, S. J., Pelphrey, K. A., Green, S. A., Bookheimer, S. Y., Dapretto, M., & GENDAAR Consortium. (2020). Sex Differences in Functional Connectivity of the Salience, Default Mode, and Central Executive Networks in Youth with ASD. Cerebral Cortex (New York, N.Y.: 1991), 30(9), 5107–5120. https://doi.org/10.1093/cercor/bhaa105. [DOI] [PMC free article] [PubMed]
- Lawrence K.E., Hernandez L.M., Eilbott J., Jack A., Aylward E., Gaab N., Van Horn J.D., Bernier R.A., Geschwind D.H., McPartland J.C., Nelson C.A., Webb S.J., Pelphrey K.A., Bookheimer S.Y., Dapretto M., Aylward E., Bernier R.A., Bookheimer S.Y., Dapretto M., Gaab N., Geschwind D.H., Jack A., McPartland J.C., Nelson C.A., Pelphrey K.A., Van Horn J.D., Webb S.J., Ankenman K., Corrigan S., Depedro-Mercier D., Guilford D., Gupta A.R., Jacokes Z., Jeste S., Keifer C.M., Kresse A., Libsack E., Lowe J.K., MacDonnell E., McDonald N., Naples A., Neuhaus E., Sullivan C.A.W., Tsapelas H., Torgerson C.M., Ventola P., Welker O., Wolf J. Neural responsivity to social rewards in autistic female youth. Transl. Psychiatry. 2020;10(1) doi: 10.1038/s41398-020-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Caverhill-Godkewitsch S., Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. NeuroImage. 2010;52(1):20–31. doi: 10.1016/j.neuroimage.2010.03.072. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Amaral D.G., Solomon M., Rogers S.J., Ozonoff S., Nordahl C.W. Sex differences in the amygdala resting-state connectome of children with autism spectrum disorder. Biol. Psychiatry Cognit. Neurosci. Neuroimag. 2020;5(3):320–329. doi: 10.1016/j.bpsc.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Andrews D.S., Ozonoff S., Solomon M., Rogers S., Amaral D.G., Nordahl C.W. Longitudinal evaluation of cerebral growth across childhood in boys and girls with autism spectrum disorder. Biol. Psychiatry. 2021;90(5):286–294. doi: 10.1016/j.biopsych.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam S.R., Libby S.J., Wing L., Gould J., Taylor C. The diagnostic interview for social and communication disorders: algorithms for ICD-10 childhood autism and Wing and Gould autistic spectrum disorder. J. Child Psychol. Psychiatry. 2002;43(3):327–342. doi: 10.1111/jcpp.2002.43.issue-310.1111/1469-7610.00024. [DOI] [PubMed] [Google Scholar]
- Lei J., Lecarie E., Jurayj J., Boland S., Sukhodolsky D.G., Ventola P., Pelphrey K.A., Jou R.J. Altered neural connectivity in females, but not males with autism: preliminary evidence for the female protective effect from a quality-controlled diffusion tensor imaging study. Autism Res. Offi. J. Int. Soc. Autism Res. 2019;12(10):1472–1483. doi: 10.1002/aur.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard, W. & Lenhard, A., 2016. Calculation of Effect Sizes. Retrieved from: https://www.psychometrica.de/effect_size.html. Dettelbach (Germany): Psychometrica. 10.13140/RG.2.2.17823.92329. [DOI]
- Lin H.-Y., Perry A., Cocchi L., Roberts J.A., Tseng W.-Y.-I., Breakspear M., Gau S.-S.-F. Development of frontoparietal connectivity predicts longitudinal symptom changes in young people with autism spectrum disorder. Transl. Psychiatry. 2019;9(1):86. doi: 10.1038/s41398-019-0418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. NeuroImage. 2011;56(3):1832–1838. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Lai M.-C., Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry. 2019;24(10):1435–1450. doi: 10.1038/s41380-018-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R., Hull L., Mandy W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(6):466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E., Leventhal B., DiLavore P., Pickles A., Rutter M. The Autism diagnostic observation schedule—Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord, C., Rutter, M., DiLavore, P. C., Risi, S., Gotham, K. O., & Bishop, S. L., 2012. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules 1–4. Western Psychological Services, Torrance.
- Maier S., Tebartz van Elst L., Perlov E., Düppers A.L., Nickel K., Fangmeier T., Endres D., Riedel A. Cortical properties of adults with autism spectrum disorder and an IQ>100. Psychiatry Res. Neuroimaging. 2018;279:8–13. doi: 10.1016/j.pscychresns.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Mandy W. In: Child Psychology and Psychiatry. Skuse D., Bruce H., Dowdney L., editors. John Wiley & Sons, Ltd; Hoboken: 2017. Autism Spectrum Disorder – An Evolving Construct; pp. 195–202. [DOI] [Google Scholar]
- Marek, S., Tervo-Clemmens, B., Calabro, F. J., Montez, D. F., Kay, B. P., Hatoum, A. S., Donohue, M. R., Foran, W., Miller, R. L., Feczko, E., Miranda-Dominguez, O., Graham, A. M., Earl, E. A., Perrone, A. J., Cordova, M., Doyle, O., Moore, L. A., Conan, G., Uriarte, J., … Dosenbach, N. U. F., 2020. Towards Reproducible Brain-Wide Association Studies. BioRxiv, 2020.08.21.257758. 10.1101/2020.08.21.257758. [DOI]
- McLaughlin K., Travers B.G., Dadalko O.I., Dean D.C., Tromp D.o., Adluru N., Destiche D., Freeman A., Prigge M.D., Froehlich A., Duffield T.C., Zielinski B.A., Bigler E.D., Lange N., Anderson J.S., Alexander A.L., Lainhart J.E. Longitudinal development of thalamic and internal capsule microstructure in autism spectrum disorder. Autism Res. Offi. J. Int. Soc. Autism Res. 2018;11(3):450–462. doi: 10.1002/aur.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei T., Llera A., Floris D.L., Forde N.J., Tillmann J., Durston S., Moessnang C., Banaschewski T., Holt R.J., Baron-Cohen S., Rausch A., Loth E., Dell’Acqua F., Charman T., Murphy D.G.M., Ecker C., Beckmann C.F., Buitelaar J.K. EU-AIMS LEAP group. Gray matter covariations and core symptoms of autism: The EU-AIMS Longitudinal European Autism Project. Mol. Autism. 2020;11(1):86. doi: 10.1186/s13229-020-00389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman S.A., Buchsbaum M.S., Young D.S., Haznedar M.M., Hollander E., Shihabuddin L., Hazlett E.A., Bralet M.-C. Increased white matter metabolic rates in autism spectrum disorder and schizophrenia. Brain Imaging Behav. 2018;12(5):1290–1305. doi: 10.1007/s11682-017-9785-9. [DOI] [PubMed] [Google Scholar]
- Moessnang C., Baumeister S., Tillmann J., Goyard D., Charman T., Ambrosino S., Baron-Cohen S., Beckmann C., Bölte S., Bours C., Crawley D., Dell’Acqua F., Durston S., Ecker C., Frouin V., Hayward H., Holt R., Johnson M., Jones E., Lai M.-C., Lombardo M.V., Mason L., Oldenhinkel M., Persico A., Cáceres A.S.José., Spooren W., Loth E., Murphy D.G.M., Buitelaar J.K., Banaschewski T., Brandeis D., Tost H., Meyer-Lindenberg A. Social brain activation during mentalizing in a large autism cohort: the longitudinal European Autism Project. Mol. Autism. 2020;11(1) doi: 10.1186/s13229-020-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H.-C., Lin H.-Y., Chen Y.-C., Tseng W.-Y.-I., Gau S.-S.-F. Boys with autism spectrum disorder have distinct cortical folding patterns underpinning impaired self-regulation: a surface-based morphometry study. Brain Imaging Behav. 2020;14(6):2464–2476. doi: 10.1007/s11682-019-00199-0. [DOI] [PubMed] [Google Scholar]
- Nordahl C.W., Iosif A.-M., Young G.S., Hechtman A., Heath B., Lee J.K., Libero L., Reinhardt V.P., Winder-Patel B., Amaral D.G., Rogers S., Solomon M., Ozonoff S. High psychopathology subgroup in young children with autism: associations with biological sex and amygdala volume. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59(12):1353–1363.e2. doi: 10.1016/j.jaac.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson E., Bedford S.A., Devenyi G.A., Patel R., Tullo S., Park M.T.M., Parent O., Anagnostou E., Baron-Cohen S., Bullmore E.T., Chura L.R., Craig M.C., Ecker C., Floris D.L., Holt R.J., Lenroot R., Lerch J.P., Lombardo M.V., Murphy D.G.M., Chakravarty M.M. Examining the Boundary Sharpness Coefficient as an Index of Cortical Microstructure in Autism Spectrum Disorder. Cerebral Cortex. 2021;31:3338–3352. doi: 10.1093/cercor/bhab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L.A., Mash L.E., Linke A., Fong C.H., Müller R.-A., Fishman I. Sex-related patterns of intrinsic functional connectivity in children and adolescents with autism spectrum disorders. Autism Int. J. Res. Pract. 2020;24(8):2190–2201. doi: 10.1177/1362361320938194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J., Bansal R., Goh S., Rodie M., Sawardekar S., Peterson B.S. Parsing the heterogeneity of brain metabolic disturbances in autism spectrum disorder. Biol. Psychiatry. 2020;87(2):174–184. doi: 10.1016/j.biopsych.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.S., Zargarian A., Peterson J.B., Goh S., Sawardekar S., Williams S.C.R., Lythgoe D.J., Zelaya F.O., Bansal R. Hyperperfusion of frontal white and subcortical gray matter in autism spectrum disorder. Biol. Psychiatry. 2019;85(7):584–595. doi: 10.1016/j.biopsych.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R.C.M., Dauvermann M.R., Whalley H.C., Baynham K., Lawrie S.M., Stanfield A.C. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci. Biobehav. Rev. 2012;36(2):901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Postema M.C., van Rooij D., Anagnostou E., Arango C., Auzias G., Behrmann M., Filho G.B., Calderoni S., Calvo R., Daly E., Deruelle C., Di Martino A., Dinstein I., Duran F.L.S., Durston S., Ecker C., Ehrlich S., Fair D., Fedor J., Feng X., Fitzgerald J., Floris D.L., Freitag C.M., Gallagher L., Glahn D.C., Gori I., Haar S., Hoekstra L., Jahanshad N., Jalbrzikowski M., Janssen J., King J.A., Kong X.Z., Lazaro L., Lerch J.P., Luna B., Martinho M.M., McGrath J., Medland S.E., Muratori F., Murphy C.M., Murphy D.G.M., O’Hearn K., Oranje B., Parellada M., Puig O., Retico A., Rosa P., Rubia K., Shook D., Taylor M.J., Tosetti M., Wallace G.L., Zhou F., Thompson P.M., Fisher S.E., Buitelaar J.K., Francks C. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-13005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge M.B.D., Lange N., Bigler E.D., King J.B., Dean D.C., Adluru N., Alexander A.L., Lainhart J.E., Zielinski B.A. A 16-year study of longitudinal volumetric brain development in males with autism. NeuroImage. 2021;236:118067. doi: 10.1016/j.neuroimage.2021.118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto A.B., Kenworthy L., Yerys B.E., Bascom J., Wieckowski A.T., White S.W., Wallace G.L., Pugliese C., Schultz R.T., Ollendick T.H., Scarpa A., Seese S., Register-Brown K., Martin A., Anthony L.G. What about the girls? Sex-based differences in autistic traits and adaptive skills. J. Autism Dev. Disord. 2018;48(5):1698–1711. doi: 10.1007/s10803-017-3413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retico A., Giuliano A., Tancredi R., Cosenza A., Apicella F., Narzisi A., Biagi L., Tosetti M., Muratori F., Calderoni S. The effect of gender on the neuroanatomy of children with autism spectrum disorders: a support vector machine case-control study. Mol. Autism. 2016;7:5. doi: 10.1186/s13229-015-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon G. Vintage Books; New York: 2019. Gender and our brains: How new neuroscience explodes the myths of the male and female minds. [Google Scholar]
- Rutter M., Le Couteur A., Lord C. Western Psychological Services; Los Angeles, CA: 2003. Autism Diagnostic Interview-Revised (ADI-R) [Google Scholar]
- Saunders A., Kirk I.J., Waldie K.E. Hemispheric coherence in ASD with and without comorbid ADHD and anxiety. Biomed Res. Int. 2016;2016:1–12. doi: 10.1155/2016/4267842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M., Kochalka J., Padmanabhan A., Supekar K., Menon V. Sex differences in cortical volume and gyrification in autism. Mol. Autism. 2015;6:42. doi: 10.1186/s13229-015-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Regenbogen C., Pauly K.D., Gossen A., Schneider D.A., Mevissen L., Michel T.M., Gur R.C., Habel U., Schneider F. Evidence for gender-specific endophenotypes in high-functioning autism spectrum disorder during empathy. Autism Res. Offi. J. Int. Soc. Autism Res. 2013;6(6):506–521. doi: 10.1002/aur.1310. [DOI] [PubMed] [Google Scholar]
- Schopler E., Reichler R.J., Renner B.R. Western Psychological Services; Los Angeles, CA: 1988. The Childhood Autism Rating Scale. [Google Scholar]
- Schumann C.M., Bloss C.S., Barnes C.C., Wideman G.M., Carper R.A., Akshoomoff N., Pierce K., Hagler D., Schork N., Lord C., Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.D., Nordahl C.W., Li D.D., Lee A., Angkustsiri K., Emerson R.W., Rogers S.J., Ozonoff S., Amaral D.G. Extra-axial cerebrospinal fluid in high-risk and normal-risk children with autism aged 2–4 years: a case-control study. Lancet Psychiatry. 2018;5(11):895–904. doi: 10.1016/S2215-0366(18)30294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds C., Sukhareva G.E. The first account of the syndrome Asperger described? Part 2: the girls. Eur. Child Adolesc. Psychiatry. 2020;29(4):549–564. doi: 10.1007/s00787-019-01371-z. [DOI] [PubMed] [Google Scholar]
- Smith R.E.W., Avery J.A., Wallace G.L., Kenworthy L., Gotts S.J., Martin A. Sex differences in resting-state functional connectivity of the cerebellum in autism spectrum disorder. Front. Hum. Neurosci. 2019;13:104. doi: 10.3389/fnhum.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J.F., van der Miesen A.I., Caplan R., Hughes C., daVanport S., Lai M.-C. Both sex- and gender-related factors should be considered in autism research and clinical practice. Autism Int. J. Res. Pract. 2020;24(3):539–543. doi: 10.1177/1362361320913192. [DOI] [PubMed] [Google Scholar]
- Subbaraju V., Suresh M.B., Sundaram S., Narasimhan S. Identifying differences in brain activities and an accurate detection of autism spectrum disorder using resting state functional-magnetic resonance imaging: A spatial filtering approach. Med. Image Anal. 2017;35:375–389. doi: 10.1016/j.media.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Sussman D., Leung R.C., Vogan V.M., Lee W., Trelle S., Lin S., Cassel D.B., Chakravarty M.M., Lerch J.P., Anagnostou E., Taylor M.J. The autism puzzle: diffuse but not pervasive neuroanatomical abnormalities in children with ASD. NeuroImage. Clin. 2015;8:170–179. doi: 10.1016/j.nicl.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Sun N., Floris D.L., Zhang X., Di Martino A., Yeo B.T.T. Reconciling dimensional and categorical models of autism heterogeneity: a brain connectomics and behavioral study. Biol. Psychiatry. 2020;87(12):1071–1082. doi: 10.1016/j.biopsych.2019.11.009. [DOI] [PubMed] [Google Scholar]
- Tillmann J., Ashwood K., Absoud M., Bölte S., Bonnet-Brilhault F., Buitelaar J.K., Calderoni S., Calvo R., Canal-Bedia R., Canitano R., De Bildt A., Gomot M., Hoekstra P.J., Kaale A., McConachie H., Murphy D.G., Narzisi A., Oosterling I., Pejovic-Milovancevic M., Persico A.M., Puig O., Roeyers H., Rommelse N., Sacco R., Scandurra V., Stanfield A.C., Zander E., Charman T. Evaluating sex and age differences in ADI-R and ADOS scores in a large european multi-site sample of individuals with autism spectrum disorder. J. Autism Dev. Disord. 2018;48(7):2490–2505. doi: 10.1007/s10803-018-3510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Reduced Local and Increased Long-Range Functional Connectivity of the Thalamus in Autism Spectrum Disorder. Cerebral Cortex. 2019;29(2):573–585. doi: 10.1093/cercor/bhx340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakoshis S., Martínez-Cañada P., Rocchi F., Canella C., You W., Chakrabarti B., Ruigrok A.NV., Bullmore E.T., Suckling J., Markicevic M., Zerbi V., Baron-Cohen S., Gozzi A., Lai M.-C., Panzeri S., Lombardo M.V. Intrinsic excitation-inhibition imbalance affects medial prefrontal cortex differently in autistic men versus women. ELife. 2020;9 doi: 10.7554/eLife.55684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers B.G., Tromp D.P.M., Adluru N., Lange N., Destiche D., Ennis C., Nielsen J.A., Froehlich A.L., Prigge M.B.D., Fletcher P., Anderson J.S., Zielinski B.A., Bigler E.D., Lainhart J.E., Alexander A.L. Atypical development of white matter microstructure of the corpus callosum in males with autism: a longitudinal investigation. Mol. Autism. 2015;6(1):15. doi: 10.1186/s13229-015-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij D., Anagnostou E., Arango C., Auzias G., Behrmann M., Busatto G.F., Calderoni S., Daly E., Deruelle C., Di Martino A., Dinstein I., Duran F.L.S., Durston S., Ecker C., Fair D., Fedor J., Fitzgerald J., Freitag C.M., Gallagher L., Gori I., Haar S., Hoekstra L., Jahanshad N., Jalbrzikowski M., Janssen J., Lerch J., Luna B., Martinho M.M., McGrath J., Muratori F., Murphy C.M., Murphy D.G.M., O’Hearn K., Oranje B., Parellada M., Retico A., Rosa P., Rubia K., Shook D., Taylor M., Thompson P.M., Tosetti M., Wallace G.L., Zhou F., Buitelaar J.K. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. Am. J. Psychiatry. 2018;175(4):359–369. doi: 10.1176/appi.ajp.2017.17010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via E., Radua J., Cardoner N., Happé F., Mataix-Cols D. Meta-analysis of gray matter abnormalities in autism spectrum disorder: Should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Arch. Gen. Psychiatry. 2011;68(4):409–418. doi: 10.1001/archgenpsychiatry.2011.27. [DOI] [PubMed] [Google Scholar]
- Werling D.M. The role of sex-differential biology in risk for autism spectrum disorder. Biol. Sex Diff. 2016;7:58. doi: 10.1186/s13293-016-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widaman K.F., Helm J.L., Castro-Schilo L., Pluess M., Stallings M.C., Belsky J. Distinguishing ordinal and disordinal interactions. Psychol. Methods. 2012;17(4):615–622. doi: 10.1037/a0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.M., Peyre H., Toro R., Beggiato A., Ramus F. Adjusting for allometric scaling in ABIDE I challenges subcortical volume differences in autism spectrum disorder. Hum. Brain Mapp. 2020;41(16):4610–4629. doi: 10.1002/hbm.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.J., Jacob S., Elison J.T. The journey to autism: Insights from neuroimaging studies of infants and toddlers. Dev. Psychopathol. 2018;30(2):479–495. doi: 10.1017/S0954579417000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2014. What do we mean by “sex” and “gender“ http://www.who.int/gender/whatisgender/en/s (accessed 12 September 2020).
- Yang J., Lee J. Different aberrant mentalizing networks in males and females with autism spectrum disorders: Evidence from resting-state functional magnetic resonance imaging. Autism Int. J. Res. Pract. 2018;22(2):134–148. doi: 10.1177/1362361316667056. [DOI] [PubMed] [Google Scholar]
- Yankowitz L.D., Herrington J.D., Yerys B.E., Pereira J.A., Pandey J., Schultz R.T. Evidence against the “normalization” prediction of the early brain overgrowth hypothesis of autism. Mol. Autism. 2020;11(1):51. doi: 10.1186/s13229-020-00353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y., Ikeda T., Hasegawa C., An K.-M., Tanaka S., Yaoi K., Iwasaki S., Saito D.N., Kumazaki H., Hiraishi H., Kikuchi M. Shorter P1m response in children with autism spectrum disorder without intellectual disabilities. Int. J. Mol. Sci. 2021;22(5):2611. doi: 10.3390/ijms22052611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma R.J.F., Moseley R.L., Holt R.J., Rughooputh N., Floris D.L., Chura L.R., Spencer M.D., Baron-Cohen S., Suckling J., Bullmore E.T., Rubinov M. Default mode hypoconnectivity underlies a sex-related autism spectrum. Biological Psychiatry Cognit. Neurosci. Neuroimag. 2016;1(4):364–371. doi: 10.1016/j.bpsc.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeestraten E.A., Gudbrandsen M.C., Daly E., de Schotten M.T., Catani M., Dell'Acqua F., Lai M.-C., Ruigrok A.N.V., Lombardo M.V., Chakrabarti B., Baron-Cohen S., Ecker C., Murphy D.G.M., Craig M.C. Sex differences in frontal lobe connectivity in adults with autism spectrum conditions. Transl. Psychiatry. 2017;7(4):e1090. doi: 10.1038/tp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Groen W., Mennes M., Greven C., Buitelaar J., Rommelse N. Revisiting subcortical brain volume correlates of autism in the ABIDE dataset: effects of age and sex. Psychol. Med. 2018;48(4):654–668. doi: 10.1017/S003329171700201X. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yuan K.-H. ISDSA Press; Granger, IN: 2018. Practical Statistical Power Analysis Using Webpower and R (Eds) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.