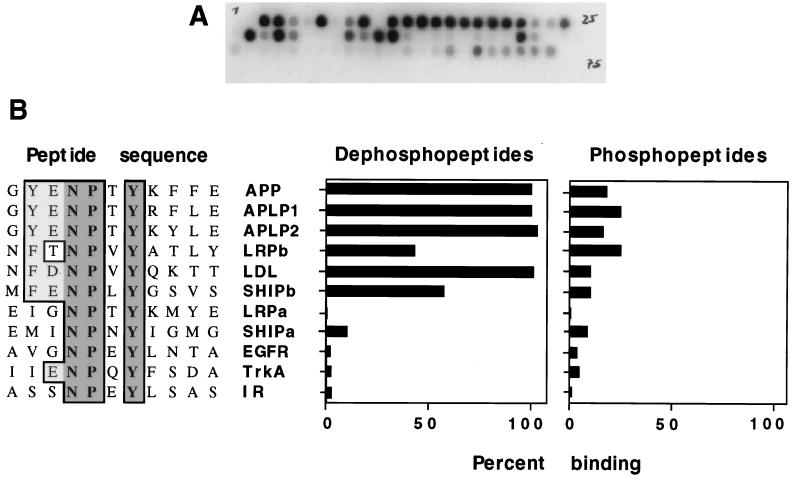
FIG. 2.
Direct binding between the Dab1 PTB domain and synthetic peptides. Various peptides, 15 to 17 residues in length, were synthesized on cellulose filters in a grided array. Filters were incubated with a 32P-labelled GSTag-PTB domain fusion protein, and the amount of the bound PTB domain was determined by autoradiography (A) or PhosphorImager (B). (A) Typical filter. (B) Comparison between interacting and noninteracting peptide sequences (left) showing invariant residues (dark shade) and conservative changes (light shade). Percent binding of the PTB domain to the indicated peptides, either unphosphorylated (left histogram) or phosphorylated (right histogram) at tyrosine, indicated by dark shading, is expressed relative to binding to the APP dephosphopeptide (set at 100%). Peptide sequences are designated by protein abbreviations. IR, insulin receptor; EGFR, epidermal growth factor receptor; LDL, low-density lipoprotein receptor.