Abstract
Study Objectives
To investigate the risk of in-hospital falls among patients receiving medications commonly used for insomnia in the hospital setting.
Methods
Retrospective cohort study of all adult hospitalizations to a large academic medical center from January, 2007 to July, 2013. We excluded patients admitted for a primary psychiatric disorder. Medication exposures of interest, defined by pharmacy charges, included benzodiazepines, non-benzodiazepine benzodiazepine receptor agonists, trazodone, atypical antipsychotics, and diphenhydramine. In-hospital falls were ascertained from an online patient safety reporting system.
Results
Among the 225,498 hospitalizations (median age = 57 years; 57.9% female) in our cohort, 84,911 (37.7%) had exposure to at least one of the five medication classes of interest; benzodiazepines were the most commonly used (23.5%), followed by diphenhydramine (8.3%), trazodone (6.6%), benzodiazepine receptor agonists (6.4%), and atypical antipsychotics (6.3%). A fall occurred in 2,427 hospitalizations (1.1%). The rate of falls per 1,000 hospital days was greater among hospitalizations with exposure to each of the medications of interest, compared to unexposed: 3.6 versus 1.7 for benzodiazepines (adjusted hazard ratio [aHR] 1.8, 95%CI 1.6–1.9); 5.4 versus 1.8 for atypical antipsychotics (aHR 1.6, 95%CI 1.4–1.8); 3.0 versus 2.0 for benzodiazepine receptor agonists (aHR 1.5, 95%CI 1.3–1.8); 3.3 versus 2.0 for trazodone (aHR 1.2, 95%CI 1.1–1.5); and 2.5 versus 2.0 for diphenhydramine (aHR 1.2, 95%CI 1.03–1.5).
Conclusions
In this large cohort of hospitalizations at an academic medical center, we found an association between each of the sedating medications examined and in-hospital falls. Benzodiazepines, benzodiazepine receptor agonists, and atypical antipsychotics had the strongest associations.
Keywords: sedatives, insomnia, falls, pharmacoepidemiology, hospitalization
Statement of Significance.
Sedating medications are widely used in hospitals to help patients sleep, but are also known to increase the risk of falls. The relative risk of falls has never been evaluated across several sedating medications in the same cohort of patients. Our study found that among the medications examined, benzodiazepines, non-benzodiazepine benzodiazepine receptor agonists (BZRAs), and atypical antipsychotics were associated with the greatest risk for falls. These findings help to inform the clinical risk-to-benefit assessment for these medications.
Introduction
Medications that depress the function of the central nervous system – sedating medications – are widely used in the inpatient setting as sleep aids. Prior studies estimate that 20–60% of patients admitted to the hospital receive pharmacological sleep aids, most commonly trazodone, benzodiazepines, and zolpidem [1–4]. While benzodiazepines have historically been the most frequently prescribed medications for insomnia, use of non-benzodiazepine medications has increased in recent years, including on-label use of non-benzodiazepine benzodiazepine receptor agonists (BZRAs), and off-label use of atypical antipsychotics for their sedative effect [2, 5–7].
While sleep disturbance is common in hospitalized patients [2, 8, 9], and places patients at higher risk for delirium and other adverse health consequences [10–12], the use of sedating medications is not without risk. A prior study found a 19% incidence of adverse drug reactions in hospitalized older adults newly prescribed zolpidem during hospitalization [13]. Prior studies have identified an increased risk of falls and fractures in patients prescribed sedating medications including zolpidem, benzodiazepines, anti-histamines, and atypical antipsychotics [14–23]. For these reasons, each of these drug classes are included in the Beers list of medications to be avoided in older adults [24].
The comparative safety of these commonly used medication classes, however, remains uncertain. The recently published clinical practice guideline by the American Academy of Sleep Medicine (AASM) on the treatment of insomnia disorder in adults calls for additional data on the efficacy and safety of these pharmaceutical agents, noting that highly variable data reporting in existing trials makes it impossible to compare efficacy or safety data from one trial to another [25]. We sought to examine the associations between five classes of medications commonly used as sleep aids in the hospital setting – benzodiazepines, non-benzodiazepine BZRAs, atypical antipsychotics, trazodone, and diphenhydramine – and in-hospital falls.
Methods
Setting and data collection
We conducted a retrospective cohort study of patients admitted to a large, urban academic medical center in Boston, Massachusetts from January 2007 through July 2013. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center and granted a waiver of informed consent. Data were collected from electronic medical information databases maintained at the medical center and supplemented by chart review where noted.
Inclusion and exclusion criteria
All hospitalizations of patients at least 18 years of age were eligible for inclusion. We excluded those admitted to a psychiatry service or with a primary or secondary discharge diagnosis of a psychotic disorder, defined by the Elixhauser comorbidity “Psychoses” which includes International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 295.00–298.9, 299.10–299.11 and Diagnosis Related Groups (DRG) 430 and 885 [26], since we were interested in the use of the medications of interest (notably, antipsychotics) for conditions other than primary psychotic disorders.
Medication exposures of interest
We investigated five different types of sedating medications commonly used for insomnia, including benzodiazepines, non-benzodiazepine BZRAs, atypical antipsychotics, trazodone, and diphenhydramine. For all medications, exposure was defined by pharmacy charges, and treated as a time-varying covariate, with each day of a given hospitalization being categorized as exposed or unexposed to each medication class of interest.
Outcome
Nurses at the medical center are required to record the occurrence of all in-hospital falls using an online patient safety reporting system, to fulfill mandatory reporting requirements to the Massachusetts Department of Public Health. We queried this system for all in-hospital falls during our cohort time frame.
Covariates
In our multivariable models, we included variables hypothesized to predict the use of sedative medications, as well as variables thought to increase the risk of falls based on prior literature and clinical grounds. These included: 1) demographic variables such as age, sex, and race (self-reported by patients at the time of admission); 2) hospitalization characteristic variables, including admitting department (medicine vs. non-medicine), whether the patient spent time in the intensive care unit (ICU), and whether they received mechanical ventilation; 3) comorbidities, including the 29 conditions specified in the Healthcare Cost and Utilization Project Comorbidity Software, version 3.7, based on the work of Elixhauser et al. [26, 27], as well as delirium, dementia, and insomnia (see Appendix Table 1 for ICD-9-CM codes pertaining to each). We included the latter because insomnia itself has been associated with the outcomes of interest [28, 29]; 4) functional measures, collected by clinical nurses on admission, including the Morse Fall Scale [30] (0–100 scale, dichotomized at 50 based on generally accepted risk categorization schemes [31]), history of falls, and visual impairment (supplemented with ICD-9-CM codes in Appendix Table 1); and 5) use of other medications that are not generally used as sleep aids, but which nonetheless have sedating properties and/or have been previously demonstrated to be associated with falls, including opioids, barbiturates, and typical antipsychotics.
Statistical analysis
A 2-sided type-I error of <0.05 was used to indicate statistical significance. All analyses were conducted using SAS software, version 9.4, Cary, NC.
We report the characteristics of hospitalizations with and without an in-hospital fall, wherein medication exposures, critical care receipt, and mechanical ventilation were treated as dichotomous variables representing exposure at any point during the hospitalization (yes/no).
We used marginal Cox-type regression models to assess the association between each medication exposure of interest and in-hospital falls, focusing on time to first fall. We treated medication exposures, ICU location of care, and mechanical ventilation as time-varying covariates by categorizing each day of the hospitalization as exposed or unexposed. We used a pre-specified exposure period of two days (day of exposure and the following day) for each medication of interest, to account for the fact that these medications are often given at the end of the day, with effects that would carry over into the next day. For the mechanical ventilation and ICU variables, we considered a patient exposed from the date of the first occurrence onward, to reflect the greater severity of illness indicated by the presence of these exposures during hospitalization. Observation time was censored at hospital discharge or 21 days after admission to avoid inclusion of outlier time, which would not be representative of the typical hospitalized patient. We assessed the unadjusted association between each medication of interest and in-hospital falls using models that included the medication of interest as the only independent variable. We assessed the independent effects of each medication of interest using models that simultaneously included all of the medications of interest and all of the variables in Table 1. For all models, we accounted for repeated hospitalizations of the same patient using a robust sandwich estimator [32].
Table 1.
Hospitalization characteristics by presence or absence of an in-hospital fall
| Characteristic – n (%) | Overall | No Fall | Fall |
|---|---|---|---|
| N = 225,498 | N = 223,071 | N = 2,427 | |
| Age | |||
| <65 | 141,393 (62.7) | 140,146 (62.8) | 1,247 (51.4) |
| 65–79 | 50,998 (22.6) | 50,271 (22.5) | 727 (30.0) |
| >79 | 33,107 (14.7) | 32,654 (14.6) | 453 (18.7) |
| Race | |||
| Black | 26,911 (11.9) | 26,648 (12.0) | 263 (10.8) |
| White | 143,180 (63.5) | 141,467 (63.4) | 1,713 (70.6) |
| Other | 55,407 (24.6) | 54,956 (24.6) | 451 (18.6) |
| Female | 130,551 (57.9) | 129,446 (58.0) | 1,105 (45.5) |
| Medicine Service | 91,917 (40.8) | 90,680 (40.7) | 1,237 (51.0) |
| Intensive Care | 36,707 (16.3) | 35,926 (16.1) | 781 (32.2) |
| Mechanical Ventilation | 10,339 (4.6) | 10,050 (4.5) | 289 (11.9) |
| Functional Measuresa | |||
| History of a fallb | 37,557 (18.3) | 36,665 (18.1) | 892 (43.2) |
| Morse fall score > 50c | 58,882 (30.3) | 57,794 (30.1) | 1,088 (58.4) |
| Visual impairmentd | 65,116 (28.9) | 64,390 (28.9) | 726 (29.9) |
| Comorbidities | |||
| Congestive heart failure | 18,012 (8.0) | 17,713 (7.9) | 299 (12.3) |
| Valvular disease | 8,660 (3.8) | 8,542 (3.8) | 118 (4.9) |
| Pulmonary circulation disease | 4,284 (1.9) | 4,201 (1.9) | 83 (3.4) |
| Peripheral vascular disease | 12,106 (5.4) | 11,923 (5.3) | 183 (7.5) |
| Paralysis | 3,573 (1.6) | 3,486 (1.6) | 87 (3.6) |
| Other neurological disorders | 12,638 (5.6) | 12,357 (5.5) | 281 (11.6) |
| Chronic pulmonary disease | 31,782 (14.1) | 31,374 (14.1) | 408 (16.8) |
| Diabetes without complications | 34,552 (15.3) | 34,089 (15.3) | 463 (19.1) |
| Diabetes with complications | 12,944 (5.7) | 12,719 (5.7) | 225 (9.3) |
| Hypothyroidism | 22,731 (10.1) | 22,453 (10.1) | 278 (11.5) |
| Renal failure | 26,715 (11.9) | 26,292 (11.8) | 423 (17.4) |
| Liver disease | 11,990 (5.3) | 11,781 (5.3) | 209 (8.6) |
| Peptic ulcer disease without bleeding | 154 (0.1) | 148 (0.1) | 6 (0.3) |
| AIDS | 1,015 (0.5) | 992 (0.4) | 23 (1.0) |
| Lymphoma | 3,384 (1.5) | 3,342 (1.5) | 42 (1.7) |
| Metastatic cancer | 8,450 (3.8) | 8,308 (3.7) | 142 (5.9) |
| Solid tumor without metastasis | 5,011 (2.2) | 4,930 (2.2) | 81 (3.3) |
| Rheumatoid arthritis/collagen vascular disease | 6,193 (2.8) | 6,118 (2.7) | 75 (3.1) |
| Coagulopathy | 10,302 (4.6) | 10,041 (4.5) | 261 (10.8) |
| Obesity | 11,728 (5.2) | 11,567 (5.2) | 161 (6.6) |
| Weight loss | 4,969 (2.2) | 4,808 (2.2) | 161 (6.6) |
| Fluid and electrolyte disorders | 34,534 (15.3) | 33,819 (15.2) | 715 (29.5) |
| Chronic blood loss anemia | 3,405 (1.5) | 3,370 (1.5) | 35 (1.4) |
| Deficiency anemias | 31,400 (13.9) | 30,901 (13.9) | 499 (20.6) |
| Alcohol abuse | 8,424 (3.7) | 8,194 (3.7) | 230 (9.5) |
| Drug abuse | 5,256 (2.3) | 5,122 (2.3) | 134 (5.5) |
| Depression | 24,748 (11.0) | 24,358 (10.9) | 390 (16.1) |
| Hypertension | 98,827 (43.8) | 97,529 (43.7) | 1,298 (53.5) |
| Delirium | 9,182 (4.1) | 8,729 (3.9) | 453 (18.7) |
| Dementia | 4,692 (2.1) | 4,579 (2.1) | 113 (4.7) |
| Insomnia | 2,261 (1.0) | 2,228 (1.0) | 33 (1.4) |
| Medication Exposures of Interest | |||
| Benzodiazepine | 53,071 (23.5) | 51,975 (23.3) | 1,096 (45.2) |
| Diphenhydramine | 18,654 (8.3) | 18,410 (8.3) | 244 (10.1) |
| Trazodone | 14,812 (6.6) | 14,556 (6.5) | 256 (10.6) |
| BZRA | 14,398 (6.4) | 14,196 (6.4) | 202 (8.3) |
| Atypical antipsychotic | 14,224 (6.3) | 13,781 (6.2) | 443 (18.3) |
| Other Medication Exposures | |||
| Barbiturate | 506 (0.2) | 493 (0.2) | 13 (0.5) |
| Opioid | 132,481 (58.8) | 131,012 (58.7) | 1,469 (60.5) |
| Typical antipsychotic | 5,366 (2.4) | 5,092 (2.3) | 274 (11.3) |
a Functional variables collected by clinical nurses on admission.
b Missing in 20,734 (9.2%).
c Missing in 31,300 (13.9%).
d Missing in 36,902 (16.4%).
For hospitalizations with missing data for any of the covariates, we used multiple imputations using the fully conditional specification method under a missing-at-random assumption [33, 34]. We chose to perform 30 imputations for each, based on a joint consideration of obtaining efficient point estimates, a good estimation of standard errors, and a potentially low power falloff [35, 36]. We gauged the performance of multiple imputations by examining covariate frequencies before and after imputation, as well as related diagnostic values such as relative efficiency and a fraction of missing information.
Subgroup analyses.
Given the altered pharmacokinetics and pharmacodynamics of medications in older adults, we tested for effect modification by age for each medication of interest by sequentially adding interaction terms in our multivariable model (testing one medication class at a time). Based on these results, we performed an analysis stratified by age, wherein we re-ran our multivariable model separately in hospitalizations of patients age 65 and older, and in those younger than age 65.
We also performed a subgroup analysis among hospitalizations without any time spent in the ICU, since we hypothesized that patients in the ICU would be at lower risk for falls as a result of being less ambulatory.
Sensitivity analyses.
Because of the observational nature of our study, differences between patients who were and were not exposed to sedating medications could confound our results. To further address this possibility, we reran our analysis in the subgroup of patients with receipt of at least one of the sedating medication classes of interest. This should serve to improve comparability of exposed and unexposed time periods in that both are coming from patients with at least some exposure to sedating medications.
Finally, because the exact time of the in-hospital fall on any given calendar day was not recorded, for exposures occurring on the same day as the fall we could not be sure that exposure preceded the outcome. Accordingly, we performed a sensitivity analysis designed to assess the impact of this potential exposure misclassification. We randomly selected 100 exposures that occurred on the same day as the fall, where there was no exposure to the same medication the day before, and reviewed the medical chart to determine which came first, allowing us to estimate the percent misclassification in this sample (i.e. the proportion in which the fall preceded the exposure). We then simulated this degree of misclassification in the overall population from which this sample was drawn, by resetting the exposure status to unexposed in a randomly selected sample of medication exposures occurring on the same day as the fall without exposure to that same medication the day before. We then reran our primary outcome model to estimate the impact of misclassification on our results.
Results
There were 230,635 hospitalizations of patients age 18 and older from January 2007 to July 2013. After excluding hospitalizations with a diagnosis code for psychosis or a psychiatric attending of record (N = 5,137), there were 225,498 hospitalizations in the analytic cohort, with a total of 1,197,822 hospital days. See Table 1 for cohort characteristics overall, and stratified by presence or absence of a fall.
Medication exposures
Among the 225,498 hospitalizations in our cohort, 84,911 (37.7%) were exposed to at least one of the five medication classes of interest, and 25,111 (11.1%) were exposed to 2 or more during the course of the hospitalization; benzodiazepines were the most commonly used (53,071 [23.5%]), followed by diphenhydramine (18,654 [8.3%]), trazodone (14,812 [6.6%]), BZRAs (14,398 [6.4%]), and atypical antipsychotics (14,224 [6.3%]). Table 2 shows medication exposures by select patient characteristics (see Appendix Table 2 for all patient characteristics). Exposure to at least one of the medication classes of interest occurred in 17,873 (47.6%) hospitalizations of patients with a history of falls, and in 26,563 (45.1%) with a Morse Fall Scale score > 50.
Table 2.
Prevalence of exposure to medications commonly used for insomnia, by select patient characteristics (see Appendix Table 1 for full patient characteristics)
| Characteristics | Total | No Exposurea | Benzodiazepine | BZRA | Trazodone | Diphenhydramine | Atypical |
|---|---|---|---|---|---|---|---|
| N | N (% of total) | N (% of total) | N (% of total) | N (% of total) | N (% of total) | N (% of total) | |
| Age | |||||||
| <65 | 141,393 | 88,231 (62.4) | 35,984 (25.5) | 9,277 (6.6) | 7,156 (5.1) | 13,788 (9.8) | 7,805 (5.5) |
| 65–79 | 50,998 | 30,622 (60.1) | 11,999 (23.5) | 3,645 (7.2) | 4,368 (8.6) | 3,640 (7.1) | 3,298 (6.5) |
| >79 | 33,107 | 21,734 (65.7) | 5,088 (15.4) | 1,476 (4.5) | 3,288 (9.9) | 1,226 (3.7) | 3,121 (9.4) |
| Race | |||||||
| Black | 26,911 | 18,339 (68.2) | 4,469 (16.6) | 1,047 (3.9) | 1,463 (5.4) | 2,420 (9.0) | 1,815 (6.7) |
| White | 143,180 | 83,521 (58.3) | 38,271 (26.7) | 10,695 (7.5) | 10,753 (7.5) | 11,967 (8.4) | 10,136 (7.1) |
| Other | 55,407 | 38,727 (69.9) | 10,331 (18.7) | 2,656 (4.8) | 2,596 (4.7) | 4,267 (7.7) | 2,273 (4.1) |
| Gender | |||||||
| Female | 130,551 | 85,055 (65.2) | 28,818 (22.1) | 6,926 (5.3) | 7,664 (5.9) | 10,883 (8.3) | 7,530 (5.8) |
| Male | 94,947 | 55,532 (58.5) | 24,253 (25.5) | 7,472 (7.9) | 7,148 (7.5) | 7,771 (8.2) | 6,694 (7.1) |
| Service | |||||||
| Medical | 91,917 | 47,193 (51.3) | 28,151 (30.6) | 7,784 (8.5) | 9,390 (10.2) | 8,873 (9.7) | 9,136 (9.9) |
| Surgical | 133,581 | 93,394 (69.9) | 24,920 (18.7) | 6,614 (5.0) | 5,422 (4.1) | 9,781 (7.3) | 5,088 (3.8) |
| Intensive Care Unit Stay | |||||||
| Yes | 36,707 | 16,417 (44.7) | 14,770 (40.2) | 2,559 (7.0) | 3,784 (10.3) | 3,511 (9.6) | 4,400 (12.0) |
| No | 188,791 | 124,170 (65.8) | 38,301 (20.3) | 11,839 (6.3) | 11,028 (5.8) | 15,143 (8.0) | 9,824 (5.2) |
| Mechanical Ventilation | |||||||
| Yes | 10,339 | 3,317 (32.1) | 5,970 (57.7) | 647 (6.3) | 1,060 (10.3) | 928 (9.0) | 1,858 (18.0) |
| No | 215,159 | 137,270 (63.8) | 47,101 (21.9) | 13,751 (6.4) | 13,752 (6.4) | 17,726 (8.2) | 12,366 (5.8) |
| Functional Measuresb | |||||||
| History of a fallc | 37,557 | 19,684 (52.4) | 11,024 (29.4) | 2,413 (6.4) | 3,948 (10.5) | 2,573 (6.9) | 4,705 (12.5) |
| Morse fall score > 50d | 58,882 | 32,319 (54.9) | 15,928 (27.1) | 3,782 (6.4) | 5,696 (9.7) | 4,423 (7.5) | 6,284 (10.7) |
| Visual impairmente | 65,116 | 38,233 (58.7) | 16,103 (24.7) | 4,965 (7.6) | 5,350 (8.2) | 6,073 (9.3) | 3,952 (6.1) |
| Comorbidities | |||||||
| Delirium | 9,182 | 3,552 (38.7) | 3,689 (40.2) | 383 (4.2) | 1,115 (12.1) | 930 (10.1) | 2,474 (26.9) |
| Dementia | 4,692 | 2,526 (53.8) | 771 (16.4) | 68 (1.5) | 490 (10.4) | 105 (2.2) | 1,407 (30.0) |
| Insomnia | 2,261 | 499 (22.1) | 1,067 (47.2) | 568 (25.1) | 603 (26.7) | 285 (12.6) | 274 (12.1) |
a No exposure to any of the medication classes of interest.
b Functional variables collected by clinical nurses on admission.
c n missing = 20,734.
d n missing = 31,300.
e n missing = 36,902.
In-hospital falls
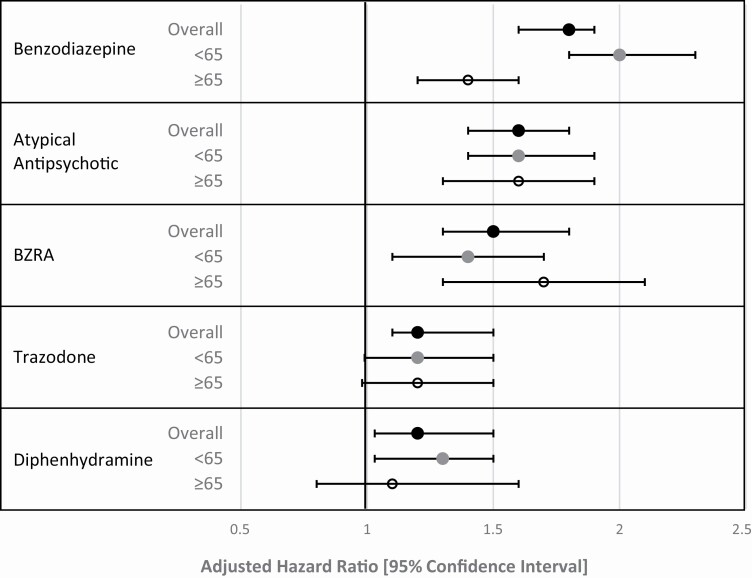
There were 2,427 falls among 225,498 hospitalizations (1.1%), with an incidence rate of 2.0 falls per 1,000 hospitalization days. For each medication of interest, the incidence of falls per 1,000 hospital days of exposure was greater than the incidence of falls per 1,000 hospital days without exposure (Table 1). In unadjusted analysis (Table 3), each medication of interest had a significant association with in-hospital falls, with the exception of diphenhydramine. After multivariable adjustment (Table 3, Figure 1), each medication had a significant association with in-hospital falls. Benzodiazepines had the strongest association (aHR 1.8, 95% CI 1.6–1.9), and trazodone (aHR 1.2, 95% CI 1.1–1.5) and diphenhydramine (aHR 1.2, 95% CI 1.03–1.5) had the weakest associations.
Table 3.
Associations between medications commonly used for insomnia and time to first in-hospital fall
| Primary Analysis | Subgroup Analysis: Non-ICUa |
Sensitivity Analysis: Any Exposureb |
Sensitivity Analysis: Exposure Misclassificationc | ||||
|---|---|---|---|---|---|---|---|
| N = 225,498 | N = 188,722 | N = 84,911 | N = 225,498 | ||||
| Falls / 1,000 Exposed Days | Falls / 1,000 Unexposed Days | Unadjustedd HR [95% CI] | Adjustede HR [95% CI] | Adjusted HR [95% CI] | Adjusted HR [95% CI] | Adjusted HR [95% CI] | |
| Benzodiazepine | 3.6 | 1.7 | 1.9 [1.8–2.1] | 1.8 [1.6–1.9] | 2.0 [1.8–2.3] | 1.9 [1.7–2.1] | 1.4 [1.2–1.5] |
| BZRA | 3.0 | 2.0 | 1.4 [1.2–1.7] | 1.5 [1.3–1.8] | 1.5 [1.3–1.8] | 1.6 [1.3–1.8] | 1.3 [1.05–1.5] |
| Trazodone | 3.3 | 2.0 | 1.5 [1.3–1.8] | 1.2 [1.1–1.5] | 1.2 [1.02–1.5] | 1.3 [1.1–1.6] | 1.0 [0.9–1.2] |
| Diphenhydramine | 2.5 | 2.0 | 1.1 [0.9–1.3] | 1.2 [1.03–1.5] | 1.1 [0.9–1.4] | 1.2 [1.02–1.5] | 1.0 [0.9–1.2] |
| Atypical antipsychotic | 5.4 | 1.8 | 2.6 [2.3–2.9] | 1.6 [1.4–1.8] | 1.5 [1.3–1.8] | 1.8 [1.6–2.0] | 1.4 [1.2–1.6] |
aSubgroup analysis excluding hospitalizations with any time spent in the intensive care unit (ICU).
bAnalysis restricted to hospitalizations with at least one exposure to a sedating medication.
cAfter reclassifying the exposure status to unexposed in a randomly selected sample of 336 (44%) of 763 medication exposures that occurred on the same day as the fall, without exposure to the same class of medications the day before.
dBased on a marginal Cox-type regression model, including only the medication of interest, modeled as a time-varying covariate, and accounting for repeated hospitalizations of the same patient using a robust sandwich estimator.
eBased on a marginal Cox-type regression model, including all variables in Table 1, including other medications, and accounting for repeated hospitalizations of the same patient using a robust sandwich estimator.
Figure 1.
Adjusted hazard ratio of falls by medication class and age (N = 225,498 overall, 141,393 age <65, and 84,105 age ≥65). Legend: Based on a marginal Cox-type regression model, including all variables in Table 1, including other medications, and accounting for repeated hospitalizations of the same patient using a robust sandwich estimator. BZRA = non-benzodiazepine benzodiazepine receptor agonists.
Subgroup analyses
A fall occurred in 1,180 (1.4%) of 84,105 hospitalizations of adults age 65 and older, with an incidence rate of 2.4 falls per 1,000 hospitalization days, and in 1,247 (0.9%) of 141,393 hospitalizations of adults less than 65, with an incidence rate of 1.7 falls per 1,000 hospitalization days. We found no evidence of effect modification by age for any of the medications of interest, with the exception of benzodiazepines, where the association was stronger in those younger than age 65, compared to those age 65 and older (p < 0.001). Table 4 and Figure 1 show the results of our fully adjusted models, stratified by age category.
Table 4.
Associations between medications commonly used for insomnia and time to first in-hospital fall, stratified by age
| Falls / 1,000 Exposed Days | Falls / 1,000 Unexposed Days | Unadjusted HRa [95% CI] | Adjusted HRb [95% CI] | |
|---|---|---|---|---|
| Age < 65 (N = 141,393) | ||||
| Benzodiazepine | 3.6 | 1.2 | 2.6 [2.3–3.0] | 2.0 [1.8–2.3] |
| BZRA | 2.7 | 1.7 | 1.4 [1.2–1.8] | 1.4 [1.1–1.7] |
| Trazodone | 3.3 | 1.7 | 1.7 [1.4–2.2] | 1.2 [0.99–1.5] |
| Diphenhydramine | 2.4 | 1.7 | 1.2 [1.02–1.5] | 1.3 [1.03–1.5] |
| Atypical antipsychotic | 5.2 | 1.6 | 2.9 [2.5–3.4] | 1.6 [1.4–1.9] |
| Age ≥ 65 (N = 84,105) | ||||
| Benzodiazepine | 3.5 | 2.3 | 1.4 [1.2–1.6] | 1.4 [1.2–1.6] |
| BZRA | 3.6 | 2.4 | 1.4 [1.1–1.8] | 1.7 [1.3–2.1] |
| Trazodone | 3.3 | 2.4 | 1.3 [1.03–1.6] | 1.2 [0.98–1.5] |
| Diphenhydramine | 2.7 | 2.4 | 1.0 [0.8–1.4] | 1.1 [0.8–1.6] |
| Atypical antipsychotic | 5.6 | 2.2 | 2.3 [2.0–2.7] | 1.6 [1.3–1.9] |
aBased on a marginal Cox-type regression model, including only the medication of interest, modeled as a time-varying covariate, and accounting for repeated hospitalizations of the same patient using a robust sandwich estimator.
bBased on a marginal Cox-type regression model, including all variables in Table 1, and accounting for repeated hospitalizations of the same patient using a robust sandwich estimator.
After excluding hospitalizations with time spent in the ICU (N = 36,776), results were similar. Benzodiazepines had the strongest association (aHR 2.0, 95% CI 1.8–2.3), and trazodone (aHR 1.2, 95% CI 1.02–1.5) and diphenhydramine (aHR 1.1, 95% CI 0.9–1.4) had the weakest, with the latter losing statistical significance.
Sensitivity analyses
Limiting the analysis to the subset of patients with exposure to at least one of the medications of interest also yielded results similar to the main analysis, with benzodiazepines having the strongest association (aHR 1.9, 95% CI 1.7–2.1), and diphenhydramine (aHR 1.2, 95% CI 1.02–1.5) and trazodone (aHR 1.3, 95% CI 1.1–1.6) the weakest (Table 3).
In total, there were 27,486 exposures to one of the medication classes of interest among patients who fell. We identified 735 medication exposures where exposure to the medication class occurred on the same day as the fall, without exposure to the same medication class the day before. Based on the review of a random selection of 100 of these exposures, we determined the percent misclassification was 44% (i.e. exposure occurred after the fall). After reclassifying the exposure status to unexposed in a randomly selected sample of 336 (44%) of these 763 medication exposures, the adjusted HRs were attenuated but qualitatively similar (Table 3), with benzodiazepines and atypical antipsychotics having the strongest associations with in-hospital falls (aHR 1.4, 95% CI 1.2–1.5 and aHR 1.4, 95% CI 1.2–1.6, respectively), and trazodone and diphenhydramine no longer having statistically significant associations with in-hospital falls (aHR 1.0, 95% CI 0.9–1.2 for both).
Discussion
In this large cohort of patients hospitalized at an academic medical center, we found an association between each of the sedating medications examined and in-hospital falls. Benzodiazepines, non-benzodiazepine BZRAs, and atypical antipsychotics had the strongest associations. These findings were robust on multiple subgroup and sensitivity analyses. To our knowledge, this is the first study to investigate the independent risks of medications commonly used for insomnia with respect to falls among hospitalized patients. Knowledge of these risks may be helpful when considering pharmacologic management of insomnia in hospitalized patients.
Several prior studies have identified a higher risk of in-hospital falls and fractures in patients prescribed sedating medications including zolpidem, benzodiazepines, anti-histamines, and atypical antipsychotics, however, they were mostly small, retrospective case-control studies [16–19, 37–40]. One large prospective study across 58 hospitals in Italy demonstrated an increased risk of falls in hospitalized patients receiving benzodiazepines or other psychotropic agents (as a grouped variable) [41]. None of these studies assessed the independent risk of falls for each of these sedating medication classes in the same cohort.
Limited data on the comparative efficacy and safety of medications commonly used for insomnia has resulted in weak and conflicting guidance around use. All of the recommendations in the recently published AASM’s Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults are graded as weak based on limitations of existing data, including small numbers of studies, small sample sizes, high risk of bias, and limited data on safety [25]. While the guideline gives a weak recommendation for the use of non-benzodiazepine BZRAs for the treatment of insomnia [25], the Beers criteria authors make a strong recommendation against the use of these medications in older adults because of their association with harms and their minimal efficacy in treating chronic insomnia [24]. Among the medications included in our analysis, trazodone is the only one not included in the Beers list as a medication to be avoided in older adults, yet the AASM guideline makes a weak recommendation against the use of trazodone, citing the paucity of existing efficacy data and the results of a single, small randomized controlled trial failing to meet their pre-specified efficacy criteria but which suggested possible harms [42]. Clinicians are resultantly in a position of deciding between medications based on weak and, at times, conflicting recommendations. Furthermore, none of the studies in the AASM guideline focus on hospitalized patients specifically; our findings highlight the critical need for studies that evaluate the efficacy of hypnotic medications for sleep in the hospitalized setting.
Our study suggests that among the medications examined, trazodone and diphenhydramine pose the least risk for falls. However, since we did not investigate other risks of these medications, including delirium and drug-drug interactions, or the effectiveness of these medications, we cannot make inferences regarding the net clinical effect on the basis of this analysis alone. We suggest considering our findings in the context of a given patient’s baseline risk profile, and existing efficacy data [25]. For example, in a young patient without a history of falls, it may be reasonable to use a benzodiazepine or non-benzodiazepine BZRA, accepting the tradeoff of higher fall risk for greater efficacy. In an older adult with a fall history, trazodone may be a better choice, accepting lower efficacy in the face of potentially lower fall risk.
The AASM guidelines further note that the limited information regarding adverse effects of hypnotic medications in older adults do not allow for meaningful conclusions about the frequency of such events in older patients compared to a younger population. Our study begins to address this question, demonstrating a similar risk of falls among older and younger adults for each of the medication classes investigated in this analysis, with the exception of benzodiazepines. While the hazard ratio of falls was higher among younger compared to older adults exposed to benzodiazepines (2.0 [1.8–2.3] versus 1.4 [1.2–1.6], respectively), the incidence was the same regardless of age (3.6 per 1,000 exposed days versus 3.5 per 1,000 exposed days, respectively), suggesting that the lower hazard ratio in older adults was attributable to the higher incidence of falls among older adults on unexposed days (1.2 per 1,000 unexposed days versus 2.3 per 1,000 unexposed days, respectively). Overall, our results suggest the associations between these medications and in-hospital falls are similar regardless of patient age.
Our sensitivity analysis to estimate the potential impact of exposure misclassification on our results yielded qualitatively similar findings, with higher risk associated with benzodiazepine, non-benzodiazepine BZRAs, and atypical antipsychotics. This subset validation exercise also revealed that in 44% of instances where exposure to the medication occurred on the same day as the fall, the fall preceded the exposure. This suggests that medications that place a patient at risk for falls are frequently given even after a fall has already occurred. This is in keeping with our finding that almost 50% of patients with a history of falls or Morse Fall Scale score >50 at the time of admission received at least one sedating medication. Although we are unable to determine the indication for which these medications were administered, these findings suggest that interventions to improve prescribing practices in this realm may be necessary.
Strengths of our study include the large sample size, use of a rigorous methodology that accounted for the time-varying nature of medication exposure, and control for more than 40 potential confounders, including demographics, the severity of illness, comorbidities, and measures of visual impairment, fall history, and the Morse Fall Scale, which are not captured in purely administrative data. Use of the patient safety reporting system to ascertain inpatient falls is also a strength, since this database is used to fulfill mandatory reporting requirements to the Department of Public Health.
There are additional limitations to our analysis. First, our study is a single-center analysis at a large, urban academic medical center, and may not be generalizable to other hospitals. Additionally, it is possible that prescribing practices and systems of care may have changed since our cohort was assembled in 2007–2013. However, the relationship between these medications and falls – the focus of our analysis – should fundamentally remain the same across time, provided confounders are addressed. While we were able to control for many factors, including those not often available in administrative data, such as the Morse Fall Scale, the observational nature of the data makes residual confounding possible. We are unable to demonstrate causality within the confines of this observational study. While a randomized controlled trial would eliminate confounding and allow for causal inference, the infrequency of falls and the number of different medications used as hypnotics make it unlikely that such a comparative safety trial will ever be conducted. The timing of the cohort also rendered us unable to examine newer medication classes for insomnia, including melatonin, melatonin receptor agonists (e.g. ramelteon), and dual orexin receptor antagonists (e.g. suvorexant), which were not yet available at our medical center during that time.
Lack of information on preadmission medication use is another limitation of our analysis, rendering us unable to examine effect modification by outpatient use. We also could not ascertain the exact timing of the fall or medication exposure on a given calendar day, and found on chart review that almost half of exposures occurring on the same day as a fall occurred after the fall. However, these exposures represented only 2.7% (735 out of 27,486) of all medication exposures among patients who fell. Further, our sensitivity analysis demonstrated qualitatively similar results. Finally, although the goal of our study was to assess the risk of falls associated with medications that are commonly used for insomnia in the hospital setting, it is likely that in some instances these medications were being used for purposes other than insomnia. The true relationship between these medications and falls should not vary based on the clinical condition for which they are being prescribed, but the possibility of confounding by indication or severity of illness exists. For example, severity of insomnia affects the risk of falls [28, 29] and may also affect the choice of sleep aid. Although we controlled for presence of insomnia and delirium in our analyses, these conditions are known to be poorly captured by administrative data, which also lacks information on severity, so this remains a limitation of our analysis.
In conclusion, in this large cohort of hospitalizations at an academic medical center, we found an association between each of the sedating medications examined and in-hospital falls. Benzodiazepines, atypical antipsychotics, and non-benzodiazepine BZRAs had the strongest associations with in-hospital falls. Effects were similar regardless of patient age. When making decisions regarding the use of sedating medications in a given patient, we suggest considering our findings in the context of the patient’s baseline risk profile, including both fall risk and risk for anticholinergic side effects (such as delirium), and the limited existing efficacy data [36].
Supplementary Material
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author Contributions
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Herzig, Rothberg, Gurwitz, Marcantonio.
Acquisition of data: Herzig, Moss, Maddaleni.
Analysis and Interpretation of data: Herzig, Rothberg, Zhou, Ngo, Bertisch, Wong, Anderson, Gurwitz, Marcantonio.
Drafting of the manuscript: Herzig.
Critical revision of the manuscript for important intellectual content: Rothberg, Zhou, Ngo, Moss, Maddaleni, Bertisch, Wong, Anderson, Gurwitz, Marcantonio.
Disclosure Statements
Financial Disclosures. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality. Dr. Marcantonio was funded by grant number K24AG035075 from the National Institute on Aging. Dr. Bertisch reports personal fees from Merck, Dohme and Sharpe, Eisai, Inc., and grants from ApniMed unrelated to the present work. The funding organizations had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Non-Financial Disclosures. None.
Conflict of interest statement. None declared.
Related paper presentations. An abstract representing an earlier version of the data contained in this manuscript was presented at the Annual Society of General Internal Medicine meeting in San Diego, April 2014.
References
- 1. Warie H, et al. . The use of hypnosedative drugs in a university hospital setting. Acta Clin Belg. 2003;58(4):225–232. [DOI] [PubMed] [Google Scholar]
- 2. Frighetto L, et al. . An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients. Health Qual Life Outcomes. 2004;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramesh M, et al. . Use of night-time benzodiazepines in an elderly inpatient population. J Clin Pharm Ther. 2002;27(2):93–97. [DOI] [PubMed] [Google Scholar]
- 4. Gillis CM, et al. . Inpatient pharmacological sleep aid utilization is common at a tertiary medical center. J Hosp Med. 2014;9(10):652–657. [DOI] [PubMed] [Google Scholar]
- 5. Herzig SJ, et al. . Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc. 2016;64(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford ES, et al. . Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the national ambulatory medical care survey 1999-2010. Sleep. 2014;37(8):1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bertisch SM, et al. . National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dobing S, et al. . Sleep quality and factors influencing self-reported sleep duration and quality in the general internal medicine inpatient population. PLoS One. 2016;11(6):e0156735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoder JC, et al. . Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arora VM, et al. . Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delaney LJ, et al. . Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review. Ann Intensive Care. 2015;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahoney JE, et al. . Zolpidem prescribing and adverse drug reactions in hospitalized general medicine patients at a veterans affairs hospital. Am J Geriatr Pharmacother. 2004;2(1):66–74. [DOI] [PubMed] [Google Scholar]
- 14. Berry SD, et al. . Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med. 2013;173(9):754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cumming RG, et al. . Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs. 2003;17(11):825–837. [DOI] [PubMed] [Google Scholar]
- 16. Chang CM, et al. . Medical conditions and medications as risk factors of falls in the inpatient older people: a case-control study. Int J Geriatr Psychiatry. 2011;26(6):602–607. [DOI] [PubMed] [Google Scholar]
- 17. Gales BJ, et al. . Relationship between the administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29(4):354–358. [DOI] [PubMed] [Google Scholar]
- 18. Kolla BP, et al. . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1–6. [DOI] [PubMed] [Google Scholar]
- 19. Rhalimi M, et al. . Medication use and increased risk of falls in hospitalized elderly patients: a retrospective, case-control study. Drugs Aging. 2009;26(10):847–852. [DOI] [PubMed] [Google Scholar]
- 20. Torstensson M, et al. . Danish register-based study on the association between specific antipsychotic drugs and fractures in elderly individuals. Age Ageing. 2017;46(2):258–264. [DOI] [PubMed] [Google Scholar]
- 21. Bakken MS, et al. . antipsychotic drugs and risk of hip fracture in people aged 60 and older in Norway. J Am Geriatr Soc. 2016;64(6):1203–1209. [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee S, et al. . Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother. 2012;10(2):83–94. [DOI] [PubMed] [Google Scholar]
- 23. Wang PS, et al. . Zolpidem use and hip fractures in older people. J Am Geriatr Soc. 2001;49(12):1685–1690. [DOI] [PubMed] [Google Scholar]
- 24. American geriatrics society 2019 updated ags beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 25. Sateia MJ, et al. . Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elixhauser A, et al. . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 27. HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP). 2013. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed March 15, 2016. [Google Scholar]
- 28. Chen TY, et al. . A greater extent of insomnia symptoms and physician-recommended sleep medication use predict fall risk in community-dwelling older adults. Sleep. 2017;40(11). doi:10.1093/sleep/zsy087 [DOI] [PubMed] [Google Scholar]
- 29. Avidan AY, et al. . Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005;53(6):955–962. [DOI] [PubMed] [Google Scholar]
- 30. Jm M, et al. . Development of a scale to identify the fall-prone patient. Can J Aging. 1989;8:366–367. [Google Scholar]
- 31. Morse JM, et al. . A prospective study to identify the fall-prone patient. Soc Sci Med. 1989;28(1):81–86. [DOI] [PubMed] [Google Scholar]
- 32. Lee, E. W., et al. . 1992. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Nato Science (Series E: Applied Sciences). vol 211. Dordrecht: Springer. doi: 10.1007/978-94-015-7983-4_14. [DOI] [Google Scholar]
- 33. Van Buuren S., et al. . Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76(12), 1049–1064. [Google Scholar]
- 34. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 35. Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? some practical clarifications of multiple imputation theory. Prev Sci Off J Soc Prev Res. 2007;8(3):206–213. [DOI] [PubMed] [Google Scholar]
- 36. Allison, P. D. 2002. Quantitative Applications in the Social Sciences: Missing data. Thousand Oaks, CA: SAGE Publications, Inc. Doi: 10.4135/9781412985079 [DOI] [Google Scholar]
- 37. Lichtenstein MJ, et al. . Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140(9):830–838. [DOI] [PubMed] [Google Scholar]
- 38. Salgado R, et al. . Factors associated with falling in elderly hospital patients. Gerontology. 1994;40(6):325–331. [DOI] [PubMed] [Google Scholar]
- 39. Chen YC, et al. . Risk factors associated with falls among Chinese hospital inpatients in Taiwan. Arch Gerontol Geriatr. 2009;48(2):132–136. [DOI] [PubMed] [Google Scholar]
- 40. Krauss MJ, et al. . A case-control study of patient, medication, and care-related risk factors for inpatient falls. J Gen Intern Med. 2005;20(2):116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Passaro A, et al. . Benzodiazepines with different half-life and falling in a hospitalized population: The GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano. J Clin Epidemiol. 2000;53(12):1222–1229. [DOI] [PubMed] [Google Scholar]
- 42. Walsh JK, et al. . Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII–R primary insomnia. Human Psychopharmacol Clin Exp. 1998;13(3):191–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.