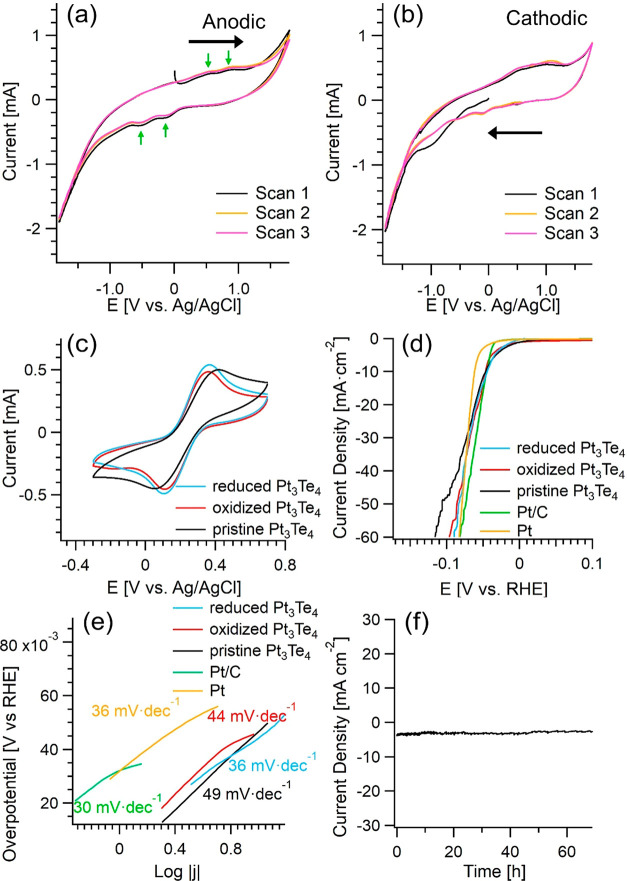
Figure 2.
(a) Anodic and (b) cathodic cyclic voltammograms of Pt3Te4 in 0.05 M phosphate-buffered saline electrolyte (pH 7.0) at a scan rate of 50 mV s–1. (c) Cyclic voltammograms of the pristine and the electrochemically treated Pt3Te4 in 0.1 M KCl solution containing 5 mM [Fe(CN)6 ]3–/4– at a scan rate of 50 mV s–1. (d) Linear sweep voltammetry curves and (e) the corresponding Tafel plots of Pt3Te4 and Pt/C catalysts in 0.5 M H2SO4 solution at a scan rate of 2 mV s–1. (f) Chronopotentiometric curve without iR correction for bulk Pt3Te4 in 0.5 M H2SO4 at a potential of −0.053 V (vs RHE).