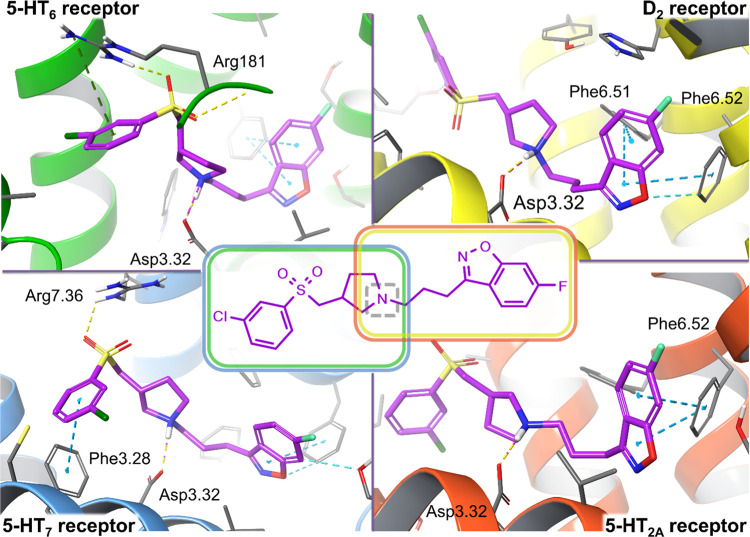
Figure 2.
Proposed binding mode of representative compound 11 with the targeted receptors. The arylsulfone fragment substituted with an alkylarylamine moiety satisfied the required interactions for both the 5-HT7 and 5-HT6 receptor binding sites (homology models based on 2RH1 and 4IAR, respectively), mimicking the interactions of their reference ligands.41−43 6-Fluoro-benzo[d]isoxazole linked to the propylamine moiety constitutes a pharmacophore with blocking properties for the 5-HT2A and D2 receptors (homology models based on 4IB4 and 3PBL, respectively).44,45 The design stage resulted in a series of 21 ligands potentially characterized by high affinity for both the desirable biological targets. Key amino acid residues engaged in ligand binding (within 4 Å from the ligand atoms) are displayed as thick sticks together with their interactions: salt bridges (dotted magenta lines), H-bonds (dotted yellow lines), halogen bonds (dotted violet lines), π–π stacking (dotted cyan lines), or cation−π (dotted green lines). The detailed complexes of lead compounds representing series I (compound 7) and series II (compound 16) are shown in Supporting Information Figures S2–S5. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.).