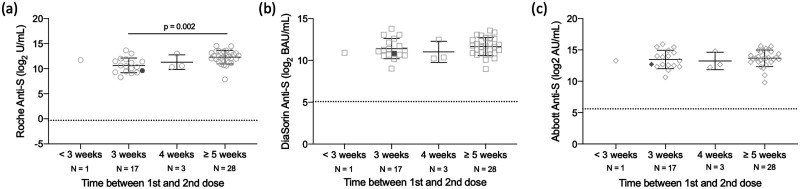
Fig. 2.
Difference in SARS-CoV-2 antibody levels by time between the first and second dose in 49 HCWs who received both the first and second dose of the Pfizer-BioNTech BNT162b2 vaccine. Samples were processed on the Roche anti-S (A), DiaSorin anti-S (B), and Abbott anti-S (C) assays. The dashed line represents the predefined assay-specific thresholds for reporting the test results as positive. Filled symbols are indicative of a participant with a prior infection and not included in statistical analysis. Statistical significance (P < 0.05) was determined using a Kruskal-Wallis test with Dunn’s multiple test correction.