Figure 1: Trial profile.
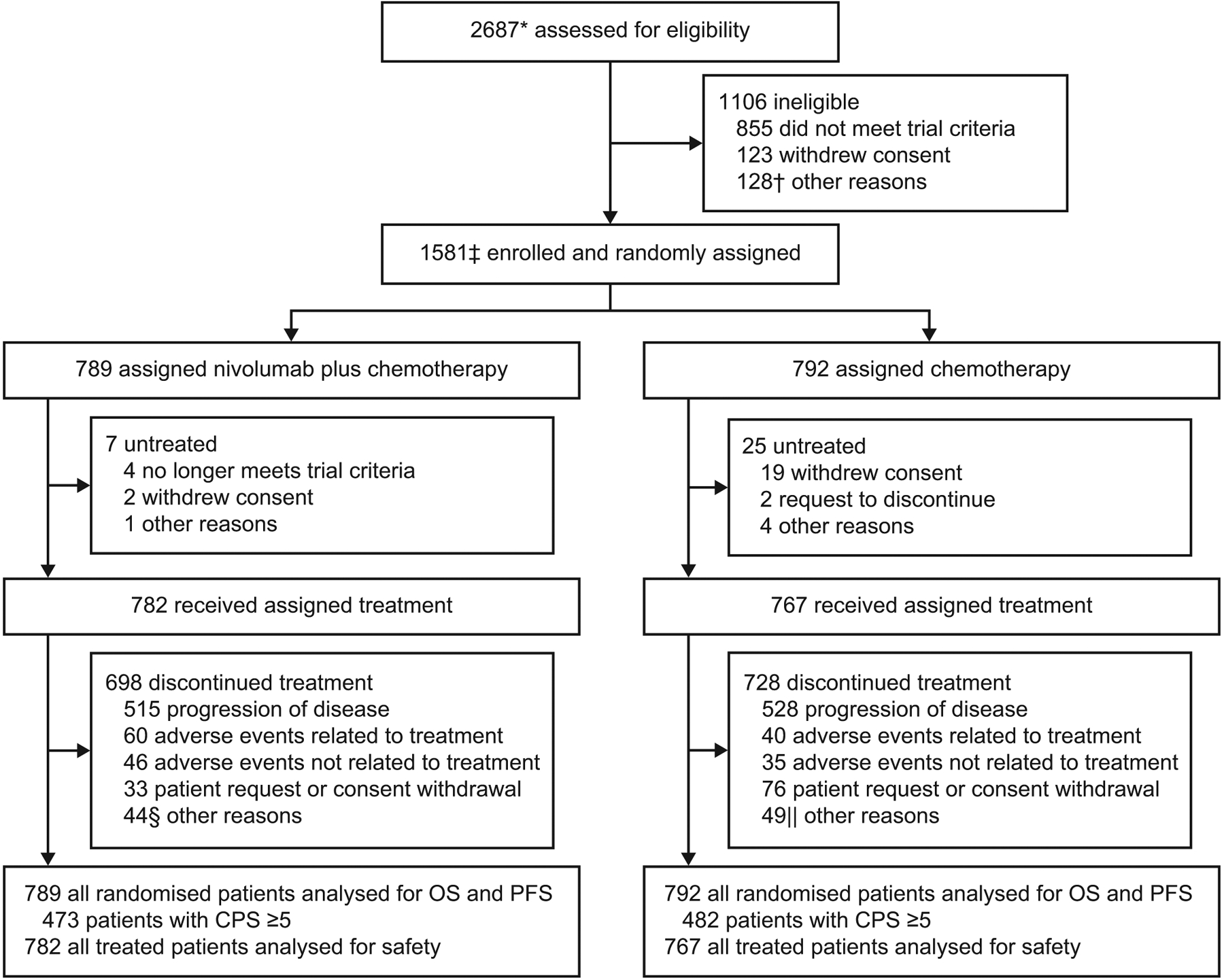
OS=overall survival. PFS=progression-free survival. CPS=combined positive score. *Enrolled patients included all concurrently randomised patients to nivolumab plus chemotherapy or chemotherapy, as well as patients enrolled before the nivolumab plus ipilimumab group was closed and not randomised to any of the treatment groups. The total number of patients randomised to three groups was 2031. The nivolumab plus ipilimumab group will remain blinded until final analysis, and the results will be reported at a later time. †Included death (n=35), adverse events (n=24), poor/noncompliance (n=15), and additional reasons (n=54). ‡Includes patients concurrently randomised to the nivolumab plus chemotherapy and chemotherapy groups. Relevant protocol deviations were noted in 21 (1%) patients. This included usage of prohibited on-treatment anti-cancer therapy (n=12), baseline ECOG PS > 1 (n=5), incorrect cancer diagnosis (n=2), and one case each of prohibited prior anti-cancer therapy (at study entry) and no baseline (measurable or evaluable) disease. §Included completion of treatment (n=20); maximum clinical benefit (n=10); two cases each of death, decline in performance, lost to follow-up, and patient relocation; and one case each of clinical worsening (hand synovitis G2), patient no longer met trial criteria, patient request to receive treatment at home, poor/noncompliance, treatment on hold due to adverse event, and unclear lung and bone lesions. ||Included maximum clinical benefit (n=25); poor/noncompliance (n=4); three cases each of patient no longer met trial criteria and death; two cases each of lost to follow-up and surgery; and one case each of bad performance status, carcinomatoses meninges, clinical progression, disease progression confirmed by central imaging (per blinded independent central review), failure after cranial progression, investigator decision, patient pursuing alternative treatment, treatment delay/discontinuation (per protocol), patient unable to tolerate treatment, and patient request to discontinue.
