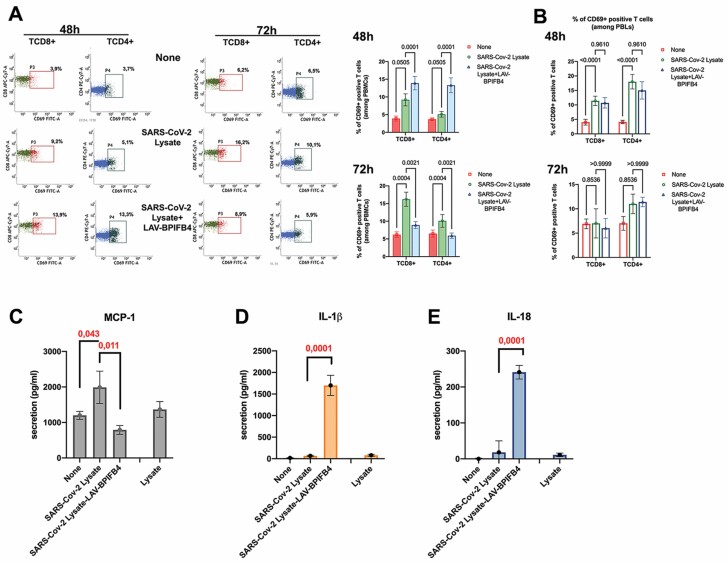
Figure 3.
Recombinant human LAV-BPIFB4 tunes inflammatory response to lysates from SARS-CoV-2-infected cells in peripheral blood mononuclear cells (PBMCs) in vitro. After a 2-hour LAV-BPIFB4 pretreatment, healthy PBMCs were incubated with SARS-CoV-2 lysate (50 μg/mL) or control lysate for 48 hours and 72 hours. In order to avoid the “activation-induced cell death” (AICD) in vitro, after the first 2 hours, RPMI-FBS medium (with or without SARS-CoV-2 lysate) was discarded and fresh RPMI-FBS medium with rhLAV-BPIFB4 (18 ng/mL) was added for the following 46 hours or 70 hours. At the end of the cell culture, supernatants were collected and PBMCs assayed by FACS analysis after staining with anti-CD3, anti-CD4, anti-CD8, and anti-CD69 mAb. (A) A representative FACS dot plot is presented. Bar graphs report the percentage ± SD of CD69+ of both CD8+ gated TCD3+ cells and CD4+ gated TCD3+ cells from 3 independent experiments using different donors (analysis of variance [ANOVA] with correction for multiple comparisons using the Holm-Sidak method). (B) Bar graphs report the percentage ± SD of CD69+ of both CD8+ gated TCD3+ cells and CD4+ gated TCD3+ cells among peripheral blood lymphocytes obtained after CD14+ immunomagnetic depletion from PBMCs in 3 independent experiments using different donors (ANOVA with correction for multiple comparisons using the Holm-Sidak method). (C–E) Multiplex ELISA assay of cytokines’ release in medium after 72 hours of treatment. Results were expressed as the mean ± SD of all sample determinations conducted in triplicate. p-values indicate significance levels comparing average cytokines’ release among different groups (ANOVA).