ABSTRACT.
We conducted a cluster-randomized trial in 48 rural villages of Ethiopia to assess the effect of community-led total sanitation (CLTS) on the diarrhea incidence of children. Twenty-four villages were randomly assigned to the intervention group and the other 24 were assigned to the control group. A CLTS intervention was implemented from January 2016 through January 2017. Baseline data collection was conducted during October and November 2015. At baseline, 906 children were recruited and followed-up until January 2017. These 906 children were randomly selected among all children in the 48 villages. To determine the 7-day period prevalence of diarrhea, four household-based surveys were conducted by independent data collectors at 3, 5, 9, and 10 months after the CLTS was initiated. To determine the incidence and longitudinal prevalence, the presence of daily diarrhea presence was recorded for 140 days using diary methods. The loss to follow-up rates were 95% for period prevalence and 93% for incidence and longitudinal prevalence. The incidence ratio and longitudinal prevalence ratio were 0.66 (95% confidence interval [CI], 0.45–0.97; P = 0.03) and 0.70 (95% CI, 0.52–0.95; P = 0.02) after adjusting for clustering and stratification. The relative risk of period prevalence was 0.66 (95% CI, 0.45–0.98; P = 0.04) at 3 months after initiation. Improved toilet coverage increased from 0.0% at baseline to 35.0% at 10 months in the intervention villages, whereas it increased from 0.7% to 2.8% in the control villages. Adherence to the intervention was comparable with that of previous studies; therefore, we suggest that the findings of this study are replicable.
BACKGROUND
Diarrhea was the third leading cause of disability-adjusted life-years (DALYs) among children younger than 5 years in 2016.1 Unsafe sanitation accounted for nearly half of the estimated 1.6 million diarrhea-specific deaths.2,3
The Sustainable Development Goals (SDGs) aim to ensure access to sanitation for all by 2030.4 However, in 2017 an estimated 673 million people were still defecating in the open, and approximately 2 billion people lacked basic sanitation facilities.5 In Ethiopia, which is one of 10 countries with the highest child death rate, diarrhea was the fourth leading cause of child death in 2015, accounting for 9% of child mortality.6 Only 7% of Ethiopians lived with access to basic or safely managed sanitation facilities in 2017.5
Recent systematic reviews have found that improved sanitation has a protective effect against diarrhea.7,8 However, the quality of the evidence was scored as low or very low. Recent trials with robust designs have found little or no effect of improved sanitation on diarrhea, with a few exceptions; therefore, the ability of sanitation interventions to reduce diarrhea has been questioned.9–15
Community-led total sanitation (CLTS) strategies, based on information indicating that merely providing toilets or subsidies did not guarantee their use and often led to problems with sustainability emerged in Bangladesh in 2000.16 With the principles of neither subsidizing toilets nor prescribing toilet models, CLTS focuses on collective behavior change to create open defecation-free villages, enabling the community to become aware of the sanitation situation and initiating the desire to improve community-wide sanitation.17 CLTS has been implemented in more than 60 countries; more than 30 of these countries, including Ethiopia, have adopted it as national policy.18
The Ethiopian government developed a CLTS and hygiene (CLTSH) policy in 2008.19 In addition to the key principle of CLTS, CLTSH emphasizes the importance of improving hygienic practices. However, it has been argued that a hygiene component, including handwashing, has been a key element of CLTS from its beginnings.20 Like CLTS, CLTSH interventions include preparation and planning (pre-initiation), initiation, post-initiation, verification, recognition, and scaling-up.
Despite the increase in CLTS, studies assessing its impact on sanitation coverage or diarrhea rates of children are still scarce. Some trials have explored the effects of India’s Total Sanitation Campaign on the health of children based on subsidies that were provided to some or all of the households in the intervention areas.10,12,21 Other trials explored the effects of an intervention combining sanitation improvements or CLTS with other components such as sanitation marketing.22–24 Trials have also investigated the effect of CLTS on toilet coverage and/or compared the effects of different approaches.25–27 The interventions analyzed during all these trials are a broad type of CLTS.18,20 We aimed to evaluate the effects of CLTS on the diarrhea rates of children in a rural area of Ethiopia with strict application of the typical principle of CLTS (i.e., no subsidy provision). Two other important characteristics of this study were that we highlighted the importance of providing improved toilets, not merely stopping open defecation, and that we measured the longitudinal prevalence and incidence of diarrhea as well as period prevalence. Although typical CLTS principles focus on ending open defecation and sanitation in low-sanitation settings, this study highlights the importance of having improved toilets that safely dispose excreta compared with the practice of open defecation and basic sanitation facilities.
METHODS
Study design.
We conducted a cluster-randomized trial in 48 rural villages (gotts) of the Cheha and the Enemor Ena Ener districts (woreda) in the Gurage zone of the Southern Nations, Nationalities, and Peoples’ Region (SNNPR) state of Ethiopia between February 1, 2015 and February 23, 2017. A phase-in design was adopted whereby a CLTS intervention was implemented in 24 intervention villages during the first phase; 24 control villages received comparable interventions during the second phase. The study was approved by the National Research Ethics Review Committee under the Ministry of Science and Technology, Federal Democratic Republic of Ethiopia (NRERC 3.10/032/2015; July 29, 2015) and the London School of Hygiene & Tropical Medicine (LSHTM Ethics Ref: 16260; February 22, 2019). This trial was registered as an International Standard Randomized Controlled Trial (ISRCTN82492848). The study protocol and rationale were published previously (Supplemental Material 1).28 The analysis used for this study adhered to the published study protocol.28
This trial was performed under the umbrella of the Integrated Water and Sanitation project funded by the Korean International Cooperation Agency (KOICA). The project comprised CLTS interventions and the provision of piped water connections from springs to communities. All components related to water improvement were implemented after the CLTS trial was completed.
Study setting.
The target areas of the project, the Cheha and Enemor Ena Ener districts, are located 185 km southwest of Addis Ababa. The populations of each district in 2014 were 133,233 and 204,937, respectively. Both districts are predominantly rural, with 90% of the land used for farming, and the major sources of income were crop production and livestock farming. Coffee, khat, and oilseeds are among the major cash crops, and eucalyptus tree plantations for income are also common.29 The Gurage ethnic group accounted for more than 80% of the population in the area, thereby giving their name to the Gurage administrative zone. The majority of the population (64%) was Muslim and 33% were Ethiopian Orthodox.29
The study areas had a specific context regarding the baseline sanitation coverage, with distinct differences from the general characteristics of low coverage described for many previous trials.10–13 The residents of the village were occasionally encouraged to build a toilet by health extension workers, particularly when visiting health centers or health posts. According to the baseline survey report, the coverage of a simple pit toilet was fairly high (73%) even before the project started, but many of the toilets were very unhygienic and poorly constructed.30 This situation involving the fairly high coverage of simple pit toilets is not atypical for many other rural settings in sub-Saharan African countries, including Kenya.9 Therefore, we highlighted the importance of improved household toilets during this trial. Open defecation based on direct observation was not especially common, and the proportion of residents who disposed their children’s feces in the toilet was high (73%).30 Handwashing practices were common before eating and before food preparation, but not after cleaning a child’s buttocks and before feeding a child (Table 1).
Table 1.
Baseline characteristics of the intervention and control groups
| Intervention | Control | |||
|---|---|---|---|---|
| N = 455 | N = 451 | |||
| n or Mean | % or SD | n or Mean | % or SD | |
| Female caregivers | 446 | 98.0% | 446 | 98.9% |
| Age of caregivers | 29.9 | 6 | 29.6 | 5.3 |
| Education of caregivers | ||||
| None | 289 | 66.4% | 289 | 66.4% |
| 1–4 grades completed | 52 | 12.0% | 64 | 14.7% |
| Male household head | 427 | 93.8% | 435 | 96.5% |
| Age of household head | 37.2 | 7.9 | 37.5 | 7.4 |
| Gurage household head | 431 | 99.1% | 433 | 99.5% |
| Religion of household head | ||||
| Muslim | 242 | 55.6% | 286 | 65.7% |
| Christian | 189 | 43.4% | 149 | 34.3% |
| Monthly income of household head, ETB | 829.7 | 641.5 | 934.6 | 739.5 |
| Female child | 226 | 49.7% | 224 | 49.7% |
| Age of child, months | 24.4 | 16.3 | 24.1 | 15.3 |
| Improved water for drinking | 319 | 72.7% | 347 | 76.9% |
| Reported handwashing practices | ||||
| Before eating | 411 | 90.3% | 388 | 86.0% |
| After defecating | 295 | 64.8% | 286 | 63.4% |
| Before food preparation | 378 | 83.1% | 356 | 78.9% |
| After cleaning child’s buttocks | 118 | 25.9% | 140 | 31.0% |
| Before feeding a child | 164 | 36.0% | 169 | 37.5% |
| Child has diarrhea, 7-day period prevalence | 101 | 22.2% | 77 | 17.1% |
| Household has a toilet, self-report | 341 | 74.9% | 364 | 80.7% |
| Toilet structure, direct observation | ||||
| Pit | 320 | 70.3% | 342 | 75.8% |
| Slab | 313 | 68.8% | 336 | 74.5% |
| Hole cover | 57 | 12.5% | 54 | 12.0% |
| Wall | 178 | 39.1% | 184 | 40.8% |
| Roof | 143 | 31.4% | 140 | 31.0% |
| Door | 35 | 7.7% | 42 | 9.3% |
| Handwashing facility with soap | 39 | 8.6% | 36 | 8.0% |
| Pit depth, m | 1.64 | 0.870 | 1.79 | 0.740 |
| Improved toilet* | 0 | 0.0% | 3 | 0.7% |
| Partially improved or better toilet† | 55 | 12.1% | 50 | 11.1% |
| Toilet utilization | ||||
| Direct observation, composite‡ | 100 | 25.2% | 109 | 24.7% |
| Cleanliness | ||||
| Presence of flies | ||||
| Presence of feces around pit hole | 76 | 16.7% | 102 | 22.6% |
| Open defecation | ||||
| Feces inside household compound | 73 | 16.0% | 63 | 14.0% |
| Feces outside household compound | 83 | 18.2% | 68 | 15.1% |
| Disposal of feces | ||||
| Into toilet | 322 | 70.8% | 340 | 75.4% |
| Open field | 76 | 16.7% | 55 | 12.2% |
ETB = Ethiopian Birr.
An improved toilet was defined as having a pit deeper than 2 m, a pit hole cover, slab, wall, door, roof, and a handwashing facility with soap.
A partially improved toilet was defined as having a pit, a pit hole cover, and slab.
Composite: presence of wet feces, footprint, and odor and absence of spiderwebs.
Participants.
A preliminary survey was conducted in August 2014 in 212 villages to assess water and sanitation coverage. We purposely selected 48 villages among the 212 villages of the two districts based on the lowest level of water and sanitation coverage. The project team performed field visits to check the accessibility of each village and excluded those that were difficult to access with a vehicle. If two selected villages were located next to each other, then we replaced one of the two with another village. The local authority requested that the same number of study villages should be allocated in every sub-district (kebele: administrative unit immediately above a village); therefore, two villages were selected from each sub-district.
We listed all households with at least one child younger than 5 years in all eligible villages and randomly selected 25 households from each village using SPSS version 21 software (IBM Corp., Armonk, NY). The average population size and number of households per village were 351 and 73, respectively. There were 3,532 households in 48 villages; of these, 1,129 (32%) had at least one child younger than 5 years.
The caregivers of selected households were visited by enumerators to register. If a caregiver was absent, then the enumerators revisited two more times. If a caregiver was absent three consecutive times or more, or if the caregiver refused to enroll in the study, then we enrolled a neighboring household. The eligibility criteria for households were the following: having a child younger than 60 months and agreeing to participate in the study by providing written informed consent. Of all the children younger than 5 years in a household, we registered only the youngest child. The study participants were recruited in the Cheha and the Enemore districts between October 17 and November 27, 2015.
Randomization and masking.
To minimize the possibility of selection bias, we identified and recruited villages before randomization. Randomization was performed during a community lottery ceremony by community leaders in each district. The allocation ratio was 1:1, with 24 villages in the intervention group and 24 villages in the control groups. If the two villages in a kebele happened to be allocated to the same arm during the lottery, then we asked community leaders to perform the lottery again until the two villages were finally assigned to different arms. Enumerators were not informed of the allocation to an intervention or control village; however, because some components of the intervention were visible, particularly toilet construction, they could not be masked to their intervention status.
Procedures.
The CLTS intervention was performed in accordance with the Ethiopian government policy from January 2016 through January 2017 (see Supplemental Text 1 for details of the intervention, such as selection criteria, demographic profiles and core tasks of CLTS promoters; benefits, training, and supervision of CLTS promoters; selection and training of CLTS facilitators; and the dates of CLTS initiation). No financial or material subsidies were provided for constructing household toilets.
A co-founder of CLTSH trained the CLTS facilitators. A team of trained CLTS facilitators comprising officials from the district health office, health professionals from health centers, and health extension workers visited the villages for pre-initiation to introduce themselves to and build rapport with the village members and to arrange the initiation schedule.
The facilitators performed the initiation process in the 24 intervention villages; this process required 1 day per village between February 11 and March 18, 2016.
The core components of the CLTS initiation process were applied with the aims of the community realizing the outcomes of open defecation practices and igniting shame or disgust.16 Village members walked through the village from one side to the other and visited open defecation sites and different types of toilets along the way and experienced the disgusting sights and smells (transect walk). Village members drew a map illustrating the sanitation situation of the village, defecation areas, and dwellings. They were asked to discuss where the dirtiest area was in their village (defecation area mapping). They calculated the amount of feces they produced per day, per week, per month, and per year, and how much they spent to treat diarrhea, dysentery, cholera, and other diseases attributable to open defecation (calculations of shit and medical expenses). They were offered a glass of water in which a hair that had touched some feces was dipped and were informed that they could ingest each other’s feces via the contaminated legs of a fly (the glass of water exercise).
At the beginning of the intervention, one or two people from each intervention village were selected as CLTS promoters to conduct post-initiation activities. If the number of households in a village was 70 or more, then two promoters were selected. Their main task was to encourage community members to build an improved toilet in their own way using locally available materials based on information gathered through community meetings and household visits. It was recommended that they should visit households every week to encourage toilet uptake. For a toilet to protect against the transmission of fecal matter, the following components were recommended: digging a pit hole with a depth of 2 m or more; installing a slab and a pit hole cover; constructing a wall, door, and roof; and installing a handwashing facility with soap. During this study, we defined an improved toilet as having all of these components. This is a more stringent definition of an improved toilet than that of the Joint Monitoring Program (JMP) of the World Health Organization (WHO)/United Nations International Children’s Emergency Fund (UNICEF).5
In principle, CLTS does not prescribe toilet types. During many CLTS interventions, particularly where open defecation practices are common, the usual approach involves convincing people to build any toilet first and then to continue to improve it.20 However, during this trial, community members in the study areas were encouraged to build improved toilets because the coverage of simple pit toilets was already high and open defecation was not as common as in many other rural settings in sub-Saharan African countries.9 Materials for toilet components were not prespecified because locally available and affordable materials could be diverse.
The CLTS facilitators and project coordinators trained CLTS promoters for 4 days in April to teach them how to build toilets, which toilet components are recommended, and the appropriate messages to deliver. After the training, the promoters promoted toilet improvement and followed-up with the toilet construction progress. The Gurage zone office, the SNNPR state of Ethiopia, and the Re-shaping Development Institute (ReDI; a development nongovernment organization based in Korea) implemented the project.
Outcomes.
The primary outcomes were the incidence, longitudinal prevalence, and 7-day period prevalence of diarrhea in children. The duration of diarrhea in children was also measured. We measured diarrhea only for the youngest child. The longitudinal prevalence and duration of diarrhea in children were recorded by caregivers using the diary method. Diarrhea calendars were distributed to 906 households in May 2016, and caregivers were asked to mark “O” or “X” on each date of the calendar according to the presence or absence, respectively, of a daily diarrhea episode. When distributing the calendar, the CLTS promoters educated the caregivers regarding how to record the daily presence of diarrhea for the registered child who was the youngest child younger than 5 years in their household at the time of enrollment. The name of the youngest child was written on every page of the calendar. Having three or more stools within 24 hours was defined as diarrhea; this was also indicated as a picture in the diarrheal calendar (Supplemental Material 2).
The CLTS promoters were trained to visit households weekly to check the recording status and encourage caregivers to continue recording correctly. CLTS promotors in the intervention villages also visited control villages to check and encourage caregivers in the same kebele to record daily diarrhea on the calendar. Apart from this encouragement to continue keeping diarrhea records, no other activities were conducted in the control villages. Data collection of daily diarrhea records was performed by independent enumerators. The 7-day prevalence of diarrhea was recorded by independent data collectors during four rounds of household surveys based on the caregiver’s recall in June, August, and December 2016, and in January 2017.
Immediately before the first round of the survey in June 2016, 36 enumerators were trained for 4 days by monitoring and evaluation specialists of the project team, district health officials, and an independent CLTS specialist who was the master trainer of CLTS. For the survey, 45 mobile devices (Y520-U22; Huawei, Shenzhen, China) were purchased and Akvo, a nonprofit software development organization based in the Netherlands, was contracted to develop an app to collect data for this particular study and to train enumerators how to use the technology. The secondary outcomes were defined as toilet coverage and toilet use. An intermediary outcome of fecal–oral contamination was also assessed by counting the number of feces inside and outside the household compound and assessing the number of flies (using a glue trap placed adjacent to the pit hole for 30 minutes). The toilet construction status was directly observed by enumerators. Each component of the toilet structures (pit, slab, pit hole cover, wall, roof, door, and handwashing facility) was photographed by enumerators during every round of the survey. To assess whether the toilet was being used, enumerators checked for the presence of a worn path to the toilet, spiderwebs at the entrance, fresh feces inside the pit, and odor. In addition, direct observations of the presence of human feces inside and outside the household compound were made.
Statistical analysis.
The sample size of the trial was calculated as follows. First, the sample size for longitudinal diarrhea prevalence was calculated based on a preliminary survey indicating that the longitudinal prevalence was 18 days per 100 child-weeks and the coefficient of variation of the true longitudinal prevalence was 0.21. Assuming 80% study power, a ratio of longitudinal prevalence of 0.79 or less, and 24 weeks of follow-up for 25 children in each village, the calculation resulted in 23 clusters per each arm.31
Second, sample size calculations for 7-day diarrheal prevalence were based on a 30% relative reduction in the intervention group and a 24% diarrhea prevalence in the comparison group that was estimated based on a preliminary survey in 2015. Assuming a type I error (α) of 0.05, 80% study power (100%*(1−β)), 20% loss to follow-up, and a coefficient of variation of 0.15, the calculations resulted in 24 clusters for each arm, each with 25 children (design effect: 2.14).22 Therefore, we selected a sample size of 600 households in each arm of 24 villages.
The assessment of the effects of CLTS on reducing diarrhea in children was conducted on an intention-to-treat basis. We used a negative binomial regression random-effects model to assess the incidence ratio and the longitudinal prevalence ratio of diarrhea; we accounted for intra-village and intra-individual correlations and adjusted for stratification by kebele. We used generalized estimating equations (GEE) with a log link and exchangeable correlation matrix to assess the relative risk (RR) of the 7-day period prevalence of diarrhea in children and 95% confidence intervals (CIs) adjusting for clustering at the village level and stratification by kebele.
Longitudinal prevalence refers to the number of days with diarrhea. For the incidence of diarrhea, we used episodes with intervals of 2 or more days. Therefore, for this study, a period of 2 or more days without diarrhea was used to distinguish one episode from another, as has been suggested to separate distinct episodes in areas where diarrhea is common.32 Therefore, two children with the same longitudinal prevalence (e.g., 7 days of diarrhea over the course of 2 weeks) could have had different diarrhea incidence rates. For example, one child could have had two episodes (e.g., for the first 2 days diarrhea occurred, followed by no diarrhea for the next 7 days, and then diarrhea occurred again for the next 5 days), whereas the other child could have had only one episode (e.g., for the first 7 days, the child had diarrhea every single day, but then the child had no diarrhea for the next 7 days). We illustrated the daily diarrhea cases of the intervention and control groups for 140 days during the CLTS intervention. The first day of the daily diarrhea records started on June 3, which was approximately 3 months after the CLTS initiation.
An adjusted analysis was not prespecified during the study protocol. We conducted sensitivity analyses by adjusting for potential residual imbalances in factors such as baseline diarrhea, the education level of the caregiver, household income level, the religion of the household head, the age of the caregiver, the age and sex of the child, and the type of drinking water source. For the adjusted analysis, we referred to a model of risk categories used by a previous study to predict diarrhea in children.33 We did not include handwashing practices and toilet utilization because we think that these variables are mediators between the variables we already included in the adjusted analysis and diarrhea.
We estimated the risk difference of secondary or intermediary outcomes (the proportion of households with an improved toilet and presence of human feces inside or outside a household compound) using ordinary least squares linear regression with robust standard errors and accounting for correlated outcomes within villages. As an additional analysis, we performed a GEE analysis to find RRs for the secondary outcomes. As a supplementary analysis, we calculated the RRs of fly counts using a negative binominal regression analysis and aggregation of fly counts around the pit hole at the village level and using the number of households with any type of toilet in a village as exposure. We performed multilevel mixed-effect linear regression to calculate the mean difference in diarrhea duration. All standard errors were adjusted for clustering.
RESULTS
A total of 1,737 households (1,301 children younger than 5 years) comprised the intervention villages and 1,795 households (1,339 children younger than 5 years) comprised the control villages at baseline.
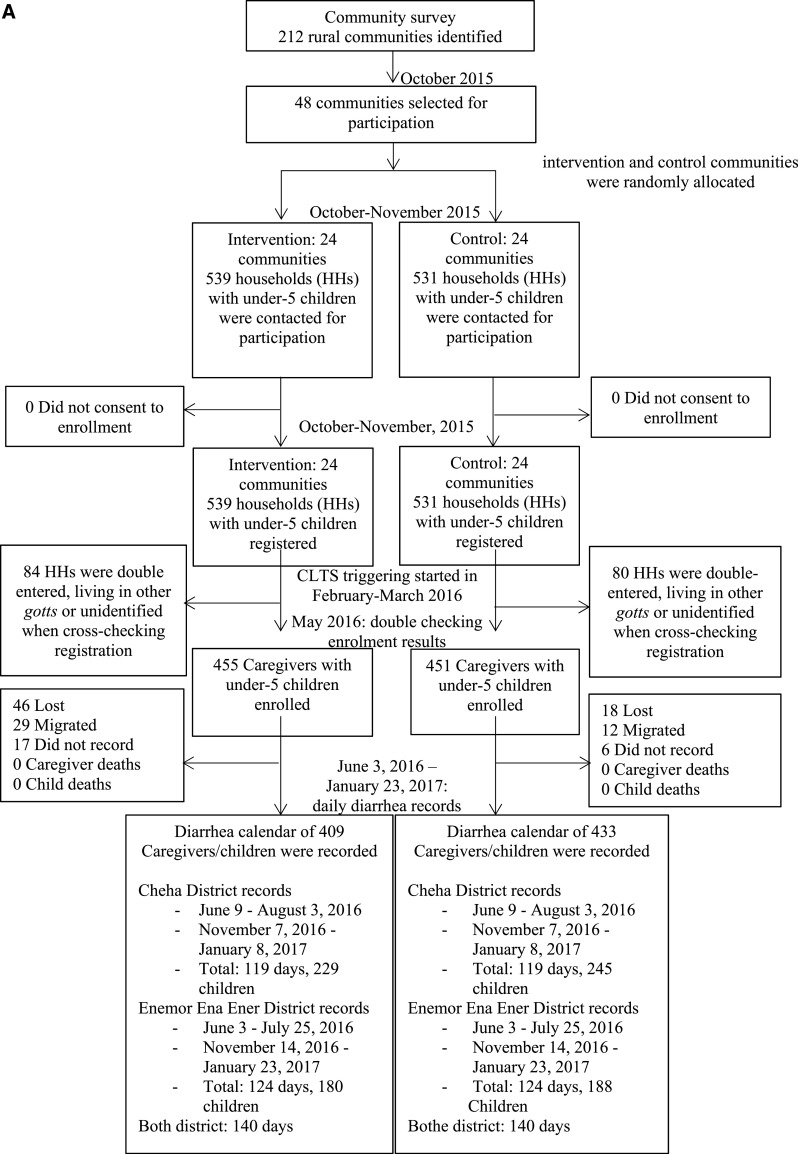
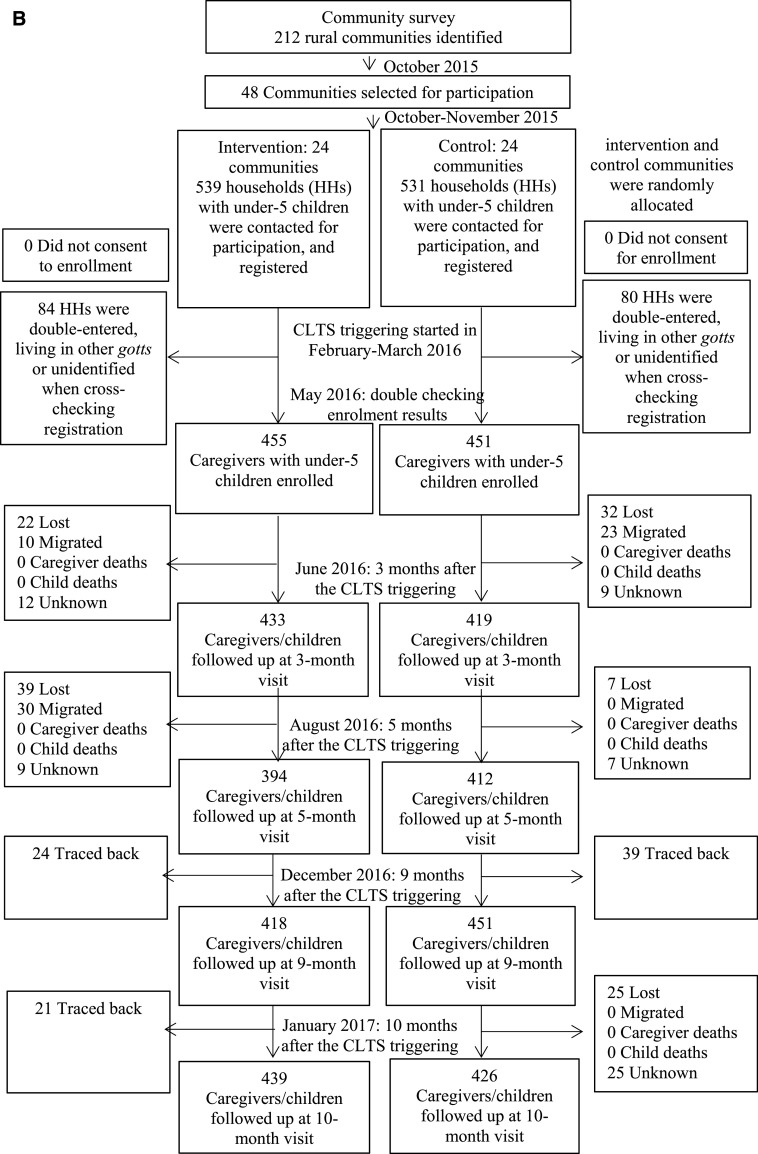
At the time of the first recruitment, the study population included 1,070 children younger than 5 years (539 in the intervention group and 530 in the control group). At the time of the second visit, to cross-check the adequacy of the registration, 84 registered children were excluded because they were living in other villages or unidentified and 80 registered children were excluded because they were double-entered (i.e., living in the same households); therefore, 906 households (mean, 25 children per village; SD, 14 children per village) remained registered (455 households from the intervention group and 451 households from the control group). We were able to follow-up 409 children (90%) and 433 children (96%) in the intervention and control groups, respectively, to determine the incidence and longitudinal prevalence of diarrhea for the full 140 days (Figure 1A). Follow-up data were collected from June 9, 2016 through January 23, 2017. For period prevalence, four rounds of follow-up surveys were performed at 3, 5, 9, and 10 months after the CLTS initiation between February 11 and March 18, 2016. A total of 439 (96%) and 426 (94%) households in the intervention and control groups, respectively, were followed-up at 10 months after the initiation (Figure 1B). We found no significant differences in socioeconomic and demographic characteristics of the caregivers and children who were retained in the trial and those who were lost to follow-up (Supplemental Table 1).
Figure 1A.
(A) Flow diagram of the longitudinal prevalence of diarrhea in children.
Figure 1B.
(B) Flow diagram of the period prevalence of diarrhea in children.
Table 1 presents the baseline characteristics of participants according to their treatment group. The socioeconomic and demographic characteristics of caregivers and household heads, as well as the handwashing practices of caregivers, were similar across groups. The coverage of improved water and sanitation was lower in the intervention group than in the control group at baseline (P = 0.02).
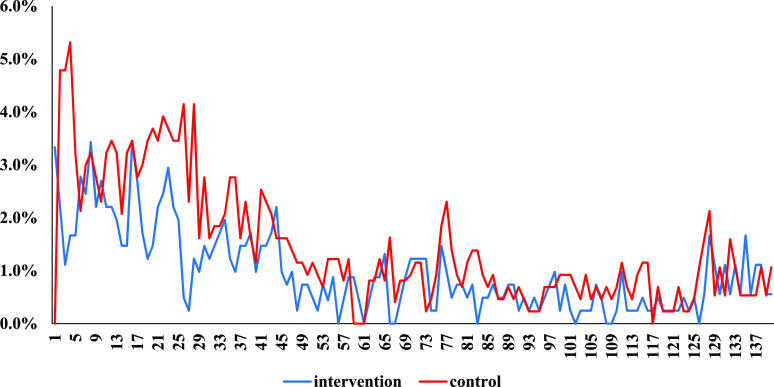
Table 2 shows the effects of the intervention on the incidence and longitudinal prevalence of diarrhea in children based on calendar records. There were 202 cases (481 days of diarrhea) in the intervention group and 298 cases (773 days of diarrhea) in the control group during the 140 days of follow-up (Table 2 and Figure 2). The corresponding incidence ratio and the longitudinal prevalence ratio were 0.66 (95% CI, 0.45–0.97; P = 0.03) and 0.70 (95% CI, 0.52–0.95; P = 0.02), respectively, after adjusting for clustering and stratification. The effects of the intervention on the duration of diarrhea in children is shown in Table 3. The results of the CLTS intervention on diarrhea duration were deemed compatible with there being no effect (95% CI, −0.8 to 0.4 days; P = 0.48).
Table 2.
Effects of the CLTS intervention on the incidence and longitudinal prevalence of diarrhea (based on calendar records)
| Intervention | Control | 95% CI | P value | |
|---|---|---|---|---|
| Total days of diarrhea | 481 (334*/147†) | 773 (551*/222†) | ||
| Total episodes | 202 (138*/64†) | 298 (220*/78†) | ||
| Total children | 409 | 433 | ||
| Person-days | 49,571 | 52,467 | ||
| Incidence (per 100 days) | 0.4 | 0.5 | ||
| Incidence ratio‡ | 0.66 | 0.45–0.97 | 0.03 | |
| Incidence ratio§ | 0.66 | 0.45–0.97 | 0.04 | |
| Longitudinal prevalence (per 100 days) | 1.0 | 1.5 | ||
| Longitudinal prevalence ratio‡ | 0.70 | 0.52–0.95 | 0.02 | |
| Longitudinal prevalence ratio§ | 0.70 | 0.51–0.95 | 0.02 |
The separated incidence and longitudinal prevalence by period are presented in Supplemental Table 2. An additional analysis adjusting for more variables is described in Supplemental Table 3.
The results for the first 62 days of the entire 140 days. The CLTS initiation was performed in February and March 2016. Starting in June 3, 2016, the presence of diarrhea in children was recorded for 140 days until January 23, 2017. There was a 3-month interval in the diary records to avoid caregiver fatigue.
The results for the next 78 days of the entire 140 days
Adjusted for clustering effect and stratification (kebele).
Adjusted for clustering effect and stratification (kebele), household head’s religion, income, caregiver’s age and education level, child’s age and sex, and type of water source.
Figure 2.
Daily prevalence of diarrhea based on calendar records (one unit of x-axis = 1 day; the first day on the x-axis is at 3 months after the CLTS initiation). This figure appears in color at www.ajtmh.org.
Table 3.
Effects of the CLTS intervention on diarrhea duration (based on calendars)
| Diarrhea episodes with an interval of ≥ 2 days | ||
|---|---|---|
| Intervention | Control | |
| Total episodes | 202 | 298 |
| Total children | 409 | 433 |
| Duration of diarrhea | ||
| 1 day | 90 (45%) | 124 (41%) |
| 2 days | 56 (28%) | 91 (31%) |
| 3 days | 32 (16%) | 36 (12%) |
| 4 days | 13 (6%) | 18 (6%) |
| > 4 days | 11 (5%) | 29 (10%) |
| Mean duration, days | 2.4 | 2.6 |
| Mean difference, days* | −0.2 | |
| 95% CI | −0.8 to 0.4 | |
| P value | 0.48 | |
| Mean difference, days† | −0.2 | |
| 95% CI | −0.8 to 0.4 | |
| P value | 0.58 | |
An additional analysis adjusting for more variables is described in Supplemental Table 4.
Adjusted for clustering effect and stratification (kebele).
Adjusted for clustering effect and stratification, household head’s religion, income, caregiver’s age and education level, child’s age and sex, and type of water source.
Table 4 shows that the 7-day period prevalence of diarrhea in children based on the caregiver’s recall decreased from 22.2% at baseline to 11.8% at the 3-month follow-up and to 7.7% at the 10-month follow-up in the intervention group. The prevalence increased from 17.1% at baseline to 17.2% and decreased to 9.9% at the same time points in the control group. The RRs of period prevalence adjusted for clustering effects and stratification were 0.66 (95% CI, 0.45–0.98; P = 0.04) at 3 months and 0.75 (95% CI, 0.35–1.60; P = 0.45) at 10 months after initiation. Pooling the four rounds of follow-up surveys indicated that the overall RR of period prevalence was 0.83 (95% CI, 0.60–1.13; P = 0.23). Except for during the first 3 months, there was no significant impact on the prevalence of diarrhea in children. All the effects on longitudinal prevalence, incidence (Supplemental Table 2), and period prevalence appeared to wane over time.
Table 4.
Effects of the CLTS intervention on the 7-day period prevalence
| Period prevalence | ||||||||
|---|---|---|---|---|---|---|---|---|
| CLTS | Control | Relative risk* | 95% CI | P | Relative risk† | 95% CI | P | |
| Overall | 0.83 | 0.60–1.13 | 0.23 | 0.78 | 0.56–1.10 | 0.16 | ||
| 3 months (June 2016) | 11.8% (51/433) | 17.2% (72/419) | 0.66 | 0.45–0.98 | 0.04 | 0.60 | 0.39–0.93 | 0.02 |
| 5 months (August 2016) | 17.3% (68/394) | 17.5% (72/412) | 0.98 | 0.68–1.39 | 0.89 | 0.89 | 0.61–1.29 | 0.54 |
| 9 months (December 2016) | 10.5% (44/418) | 11.8% (53/451) | 0.87 | 0.52–1.48 | 0.62 | 0.87 | 0.50–1.49 | 0.61 |
| 10 months (January 2017) | 7.7% (34/439) | 9.9% (42/426) | 0.75 | 0.35–1.60 | 0.45 | 0.63 | 0.28–1.43 | 0.27 |
An additional analysis adjusting for more variables is described in Supplemental Table 5.
Adjusted for clustering effect, stratification (kebele).
Adjusted for clustering effect, stratification (kebele), baseline prevalence of diarrhea, household head’s religion, income, caregiver’s age and education level, child’s age and sex, and type of water source.
Table 5 shows that the mean proportion of households with an improved toilet increased from 0.0% at baseline to 35.0% at 10 months after the CLTS initiation in the intervention group villages; however, it increased from 0.5% to 2.8% in the control group villages (risk difference, 32.3%; 95% CI, 19.1–45.4%; P < 0.001; see Supplemental Tables 6 and 7 for the results at 3, 5, 9, and 10 months).
Table 5.
Effects of the CLTS intervention on secondary and intermediate outcomes
| Survey period | 10 months after the CLTS initiation (January 2017) | ||||
|---|---|---|---|---|---|
| Intervention | Control | RD/RR | 95% CI | P | |
| Outcomes | (N = 439) | (N = 426) | |||
| Having a household toilet | 437 (99.5%) | 387 (90.8%) | 8.7% | 3.8–13.6% | < 0.001 |
| All types of toilet | |||||
| Improved toilet* | 154 (35.0%) | 12 (2.8%) | 32.3% | 19.1–45.4% | < 0.001 |
| Partially improved toilet or better† | 302 (69.0%) | 64 (15.0%) | 53.8% | 43.2–64.3% | < 0.001 |
| Hand washing facility | 207 (47.2%) | 49 (11.5%) | 35.6% | 19.5–51.7% | < 0.001 |
| Toilet utilization/self-report | 437 (99.5%) | 387 (90.8%) | 8.7% | 3.8–13.6% | < 0.001 |
| Toilet utilization/use | |||||
| Direct observations, composite‡ | 162 (36.9%) | 191 (44.8%) | −8.6% | −32.8% to 15.6% | 0.47 |
| Feces around pit hole | 63 (14.4%) | 100 (25.8%) | −11.4% | −28.8% to −5.9% | 0.20 |
| Feces in the compound | 7 (1.6%) | 30 (7.4%) | −5.4% | −11.8% to 0.9% | 0.09 |
| Feces outside compound | 5 (1.1%) | 24 (5.6%) | −4.5% | −9.4% to 0.4% | 0.07 |
| Fly number | 3.9 (6.9) | 7.6 (7.9) | 0.39 | 0.31–0.49 | < 0.001 |
| Child feces disposal | 436 (99.3%) | 384 (90.10%) | 2.6% | −0.9% to 6.1% | 0.14 |
| Reported handwashing at five critical times | 194 (44.2%) | 143 (33.6%) | 10.6% | −12.6% to 33.8% | 0.37 |
RD = risk difference; RR = risk ratio for fly counts (aggregated at village level). See Supplemental Table 6 for the results at 3, 5, and 9 months. See Supplemental Table 7 for the relative risk at 3, 5, 9, and 10 months.
An improved toilet was defined as having a pit deeper than 2 m, pit hole cover, slab, wall, door, roof, and a handwashing facility with soap. Toilet depth was not measured at the 5-month follow-up; therefore, the proportion of improved toilets was not assessed
A partially improved toilet was defined as having a pit, pit hole cover, and slab. This row includes both improved and partially improved toilets.
Composite: presence of wet feces, footprint, and odor and absence of spiderwebs.
At the 10-month follow-up, 4 of the 24 intervention villages had improved toilet coverage of 70% or greater. In the control villages, no community had coverage of 30% or greater. Ownership of a partially improved household toilet (defined as having a pit, pit hole cover, and slab) in this study increased from 11.9% at baseline to 69.0% at 10 months after the CLTS initiation in the intervention group compared with the corresponding rates of 11.6% at baseline and 15.0% at follow-up in the control group (risk difference, 53.8%; 95% CI, 43.2–64.3%; P < 0.001). The coverage of any type of toilet was already high at baseline and continued to increase in both arms. Based on direct observations, the coverage of any type of toilet at baseline in the intervention villages was 70.3%, and it increased to 99.5% at 10 months after the CLTS initiation; the corresponding values in the control group were 75.8% at baseline and 90.8% at 10 months after the CLTS initiation.
During the 10-month follow-up, all caregivers who had any type of toilet in the intervention and control groups reported that they were using the toilet (99.5% versus 90.8% in the intervention group and control group, respectively; risk difference, 8.7%; 95% CI, 3.8–13.6%; P < 0.001). The toilet utilization rate based on direct observations, however, was far less than that based on self-reports. We found no consistent pattern of the effect of the intervention on toilet utilization (Supplemental Table 6). Toilet utilization was not significantly different at 10 months after the CLTS initiation (January 2017) between the two groups based on the composite indicator comprising the presence of wet feces, footprints, and odor and absence of spiderwebs. The intervention had an effect on contamination pathways, as shown by some indicators. We recorded a declining trend in the presence of feces inside and in the immediate surroundings of the household compound (within 10 feet) in the intervention group compared with the control group. The fly count also decreased in the intervention group compared with that of the control group. The proportion of households with human feces inside the household compound decreased from 16% at baseline to 1.6% in the intervention group; however, it decreased from 14% to 7.4% in the control group (risk difference, −6.1%; 95% CI, −11.4% to −0.8%; P = 0.03) at the 9-month follow-up (Supplemental Table 6). The number of flies around the pit hole of a toilet was fewer in the intervention group than in the control group (RR, 0.60, 95% CI, 0.45–0.78, and P < 0.001 at 9 months; RR, 0.39, 95% CI, 0.31–0.49, and P < 0.001 at 10 months).
DISCUSSION
Our findings provide evidence that the CLTS intervention in rural areas of Ethiopia reduced the incidence and longitudinal prevalence of diarrhea in children and increased the coverage of an improved household toilet from 3 to 10 months after CLTS initiation. This study also found that the sanitation intervention reduced exposure to transmission pathways of fecal–oral contamination in terms of fly count. However, there was no clear evidence of the effect on 7-day period prevalence over longer follow-up durations beyond 3 months and the duration of diarrhea. We also detected no effect on the use of household toilets.
The effect size of the CLTS interventions in this study was consistent with that of recent systematic reviews of the effect of sanitation improvements on diarrhea in children (e.g., RR, 0.75; 95% CI, 0.63–0.88; P < 0.001 reported by the latest study).7,8
Our results build on those of previous trials and explain the lack of impact during previous trials of sanitation interventions.9–13 The majority of previous trials reporting no effects of sanitation improvements suggested that the absence of an effect might have been caused by insufficient coverage and use of toilets.9–13 Previous studies suggested that the absence of an effect of a sanitation intervention could be explained by the possibility that household sanitation improvements alone were insufficient to mitigate transmission of fecal pathogens, or that the toilets were ineffective at containing excreta.9,11 In particular, previous researchers expressed concerns that handwashing practices, food hygiene, and protection against contamination of animal feces could not be managed solely by improving household sanitation. The importance of these components cannot be overstated; however, this study suggests that improvements in household sanitation alone could have protective benefits against diarrhea in children. Interestingly, a profound effect of a sanitation intervention was reported in this study even though the proportion of toilet use was not different between the treatment arms. However, the absence of a difference in the use of any type of toilet between the groups indicated that the rate of use of an improved or a partially improved toilet was higher in the intervention arm than in the control arm. The fact that there was near-universal coverage of any type of toilet and a sizeable proportion of quality toilets, or at least partially improved toilets, and more widespread use of improved toilets might have contributed to reducing diarrhea. We believe that the improved toilet status was one of the most plausible factors contributing to diarrhea reduction. Clear evidence in this regard is the significant reduction in the fly count around pit holes in the intervention group compared with the control group. This finding is consistent with the results of a previous study that reported that the incidence ratio of diarrhea was 0.77 (95% CI, 0.67–0.89; P = 0.007) during the fly seasons after controlling flies compared with the control group.34
In addition, the increase in the proportions of household toilets with slab or handwashing facilities in the intervention group might have reduced the possibility of contact with feces via hands or feet, although these proportions were not measured. The handwashing practices of caregivers after defecating were somewhat better in the intervention group at 3 months after the CLTS initiation (Supplemental Table 8). Furthermore, the handwashing practices of caregivers at five critical times (before eating, after defecating, before food preparation, after cleaning a child’s buttocks, and before feeding a child) tended to be slightly higher compared with those in the control group. However, the difference was minimal. The decrease in the presence of human feces inside or outside the household compound could also be another reason for diarrhea reduction in the intervention group. Similarly, animals might have been less likely to transmit pathogens of human feces in the intervention group because of the increase in the coverage of toilets with a wall.35–37 Another reason for the absence of an effect during other trials may have been related to the frequency of measurements.32 During previous trials, diarrhea measurements were performed only once or a few times.9–11,13 A typical measurement point during previous trials was 12 months after the intervention. We assessed diarrhea cases throughout the rainy (June–August) and dry seasons. Diarrhea-related illness was measured at 140 time points from 3 months to 10 months after the CLTS initiation. If we had assessed diarrhea prevalence only at 10 months after the initiation, then we would not have been able to detect the effects of the CLTS intervention during this study.
Trials in Kenya and Zimbabwe encouraged households to shift from unimproved to improved sanitation.9,13 However, during those studies, the investigators applied a compound-based approach rather than a community-based intervention, and the community-wide coverage of improved toilets remained low at follow-up, even though the rate was very high among the households that received the intervention. Although Ngure et al. argued that children younger than 2 years are mostly exposed to fecal contamination within household compounds, this tendency might be highly context-dependent; therefore, a compound-level sanitation intervention might not be sufficient to protect children from exposure to fecal contamination, particularly when only a small proportion of households in a community receives a sanitation intervention, as in the trials in Kenya and Zimbabwe.9,13,38 Herd protection from sanitation interventions or external effects of community-wide sanitation coverage was suggested by previous studies.39,40
During the first CLTS trial to assess an effect on diarrhea in children, the proportion of households with a household toilet was 65% in the intervention group, but most of the toilets were unimproved.11 During our study, the proportion of household toilets with a slab reached 99% at 10 months after the initiation (Supplemental Table 6). We found a consistent pattern of a smaller number of feces around holes and lower fly counts in improved toilets compared with unimproved toilets within the villages that received the intervention (Supplemental Table 9). It is worth noting that even the partially improved toilets in this study were categorized as an improved toilet according to the WHO/UNICEF Joint Monitoring Program.5 It has been hypothesized that sanitation coverage must be greater than a certain threshold for adequate prevention of transmission of pathogens in diarrhea. We think that the effect of the CLTS intervention on the reduction in diarrhea in children during this study might have been caused by nearly universal coverage of improved toilets based on the WHO/UNICEF definition.5
A substantial reduction in cases of diarrhea in children was observed over time in the control group. A possible explanation was contamination of the intervention; improvements in sanitation coverage and transmission pathways were observed in the control group (e.g., the presence of feces inside or outside a household compound, ownership of an improved or partially improved toilet, safe disposal of children’s feces). Another possible explanation for the reduction in diarrhea cases in the control villages is the reduced risk of diarrhea-related illness during the dry season (September– May), although opposing findings have been reported.41,42
Daily diarrhea episodes decreased over time in the intervention and the control groups. We inferred that seasonal variation and the increased coverage of partially improved toilets, even in the control group, could have contributed to this change. We noticed that the reduction in diarrhea in the intervention group was more substantial during the earlier period of the CLTS intervention in terms of longitudinal prevalence and incidence (Supplemental Table 2). This is consistent with the fact that the coverage of improved and partially improved toilets increased more substantially during the early period of the intervention, as shown by increases of 26.6% and 42.4% in improved and at least partially improved toilet coverage for the first 3 months compared with increases of 8.4% and 14.5% during the next 7-month period (Supplemental Table 6).
This study had several limitations. First, we relied on caregivers’ reports or records of diarrhea in children and did not conduct molecular measurements of infection; therefore, we cannot rule out the possibility of bias. The inability to mask the CLTS intervention was another limitation. Adequate disposal of a child’s feces or handwashing behavior might have been overstated. Similarly, the self-reported use of a household toilet was higher than the results of direct observations. To overcome the social desirability bias, we relied on the results of direct observations of toilet construction and utilization. CLTS promoters in the intervention arm might have been a potential contamination channel in the control group through their monthly checks and follow-up of diarrhea diaries. The increases in partially improved toilets and handwashing facilities in the control villages could be partially explained by this factor.
This study reported that a number of indicators had improved more at the 10-month follow-up than at earlier times after the initiation, and it may be possible that the effects on the incidence and the longitudinal prevalence of diarrhea in children could have been more pronounced with a longer follow-up period if post-initiation activities had continued. This possibility is especially compelling because the households with partially improved toilets were in the process of constructing improved household toilets.43,44 However, this does not guarantee that the outcomes can be sustained after initiation activities are stopped . Previous studies suggested that the effects of an intervention may wane over time, particularly beyond 1 year after an intervention.9 We suggest institutionalizing a routine system for continued sanitation improvement after CLTS initiation that can be performed by government officials and community health workers.44
Adherence to the intervention in terms of ownership of an improved or partially improved toilet was comparable to that of the majority of previous studies. However, caution is needed when interpreting the generalizability of this study. This trial was performed in rural areas with high coverage of simple pit toilets and a low proportion of open defecation practices even before the CLTS intervention. Highlighting improved toilets from the onset of CLTS interventions might be difficult in other contexts, particularly those where open defecation practices are rampant and toilet coverage is low. The relatively small number of clusters in this study compared with other previous trials might have helped in the implementation of an intensive intervention, particularly during the post-initiation period. Therefore, it remains unanswered whether the findings of this study are replicable and relevant for large-scale interventions, and whether similar improvements and effects can be achieved at scale. Still, this trial provides evidence that CLTS interventions with an emphasis on improved household toilets are likely to reduce diarrhea in children. Importantly, we reduced barriers against toilet construction by encouraging community members to use locally available and affordable materials and did not compromise the improved status of toilets or neglect the principle of no financial or material subsidies.
ACKNOWLEDGMENTS
We thank the project team members for their efforts and contributions to improving water, sanitation, and hygiene in the SNNPR state of Ethiopia. We extend our appreciation to the CLTS promoters, Health Extension Workers, and District Health Directors of Enemore Ena Ener and Cheha districts.
Note: Supplemental files appear at www.ajtmh.org.
REFERENCES
- 1.Troeger Cet al. 2018. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrheal diseases in children younger than 5 years. Lancet Glob Health, 6: e255–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Risk Factor Collaborators, 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1923–1994. [DOI] [PMC free article] [PubMed]
- 3.GBD 2017 Causes of Death Collaborators, 2018. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Glob Health 392: 1736–1788. [DOI] [PMC free article] [PubMed]
- 4.UN , 2015. Transforming Our World: The 2030 Agenda for Sustainable Development. New York, NY: United Nations. [Google Scholar]
- 5.WHO/UNICEF , 2019. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 6.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE, 2016. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388: 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf Jet al. , 2018. Impact of drinking water, sanitation and handwashing with soap on childhood diarrheal disease: updated meta-analysis and meta-regression. Trop Med Int Health 23: 508–525. [DOI] [PubMed] [Google Scholar]
- 8.Freeman MCet al. , 2017. The impact of sanitation on infectious disease and nutritional status: a systematic review and meta-analysis. Int J Hyg Environ Health 220: 928–949. [DOI] [PubMed] [Google Scholar]
- 9.Null Cet al. , 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhea and child growth in rural Kenya: a cluster-randomized controlled trial. Lancet Glob Health 6: e316–e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clasen Tet al. , 2014. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Glob Health 2: e645–e653. [DOI] [PubMed] [Google Scholar]
- 11.Pickering AJ, Djebbari H, Lopez C, Coulibaly M, Alzua ML, 2015. Effect of a community-led sanitation intervention on child diarrhea and child growth in rural Mali: a cluster-randomized controlled trial. Lancet Glob Health 3: e701–e711. [DOI] [PubMed] [Google Scholar]
- 12.Patil SR, Arnold BF, Salvatore AL, Briceno B, Ganguly S, Colford JM, Gertler PJ, 2014. The effect of India’s total sanitation campaign on defecation behaviors and child health in rural Madhya Pradesh: a cluster randomized controlled trial. PLoS Med 11: e1001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey JHet al. , 2019. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomized trial. Lancet Glob Health 7: e132–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luby SPet al. , 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhea and child growth in rural Bangladesh: a cluster randomized controlled trial. Lancet Glob Health 6: e302–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammer J, Spears D, 2016. Village sanitation and child health: effects and external validity in a randomized field experiment in rural India. J Health Econ 48: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkataramanan V, Crocker J, Karon A, Bartram J, 2018. Community-led total sanitation: a mixed-methods systematic review of evidence and its quality. Environ Health Perspect 126: 026001. [DOI] [PMC free article] [PubMed]
- 17.Kar K, Chamber R, 2008. CLTS Handbook. London, United Kingdom: Plan UK. [Google Scholar]
- 18.Bongartz P, Vernon N, Fox J, 2016. Sustainable Sanitation for All. Rugby, United Kingdom: Practical Action. [Google Scholar]
- 19.FMOH , 2012. Implementing Guideline for CLTSH Programming. Addis Ababa, Ethiopia: Federal Ministry of Health. [Google Scholar]
- 20.Kar K, 2019. Scaling-up Community Led Total Sanitation: From Village to Nation. Rugby, United Kingdom: Practical Action. [Google Scholar]
- 21.Dickinson KL, Patil SR, Pattanayak SK, Poulos C, Yang J-H, 2015. Nature’s call: Impacts of sanitation choices in Orissa, India. Econ Dev Cult Change 64: 1–29. [Google Scholar]
- 22.Briceño B, Coville A, Martinez S, 2015. Promoting Handwashing and Sanitation: Evidence from a Large-Scale Randomized Trial in Rural Tanzania. Policy Research Working Paper 7164. Washington, DC: World Bank.
- 23.Borja-Vega C, 2014. The effects of the Total Sanitation and Sanitation Marketing programme on gender and ethnic groups in Indonesia. Waterlines 33: 55–70. [Google Scholar]
- 24.Cameron L, Shah M, Olivia S, 2013. Impact Evaluation of a Large-scale Rural Sanitation Project in Indonesia. Policy Research Working Paper 6360. Washington, DC: World Bank.
- 25.Crocker J, Geremew A, Atalie F, Yetie M, Bartram J, 2016. Teachers and sanitation promotion: an assessment of community-led total sanitation in Ethiopia. Environ Sci Technol 50: 6517–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guiteras R, Levinsohn J, Mobarak AM, 2015. Encouraging sanitation investment in the developing world: a cluster-randomized trial. Science 348: 903–906. [DOI] [PubMed] [Google Scholar]
- 27.Pattanayak SKet al. , 2009. Shame or subsidy revisited: social mobilization for sanitation in Orissa, India. Bull World Health Organ 87: 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung S, Doh YA, Bizuneh DB, Beyene H, Seong J, Kwon H, Kim Y, Habteyes GN, Tefera Y, Cha S, 2016. The effects of improved sanitation on diarrheal prevalence, incidence, and duration in children under five in the SNNPR State, Ethiopia: study protocol for a randomized controlled trial. Trials 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SNNPR , 2014. Demographic and Health Statistics Report of SNNPR. Hawassa: Southern Nations, Nationalities, and Peoples' Regional Office. [Google Scholar]
- 30.BDS-CDR , 2015. Baseline Survey Report: The Project of Improvement of Water Supply and Sanitation in Gurage Zone, SNNPR, Ethiopia. Addis Ababa: BDS-Center for Development Research.
- 31.Hayes RJ, Bennett S, 1999. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol 28: 319–326. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt WP, Arnold BF, Boisson S, Genser B, Luby SP, Barreto ML, Clasen T, Cairncross S, 2011. Epidemiological methods in diarrhea studies–an update. Int J Epidemiol 40: 1678–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sima LC, Reuben NG, Elimelech M, 2013. Modeling risk categories to predict the longitudinal prevalence of childhood diarrhea in Indonesia. Am J Trop Med Hyg 89: 884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavasse DC, Shier RP, Murphy OA, Huttly SR, Cousens SN, Akhtar T, 1999. Impact of fly control on childhood diarrhoea in Pakistan: community-randomised trial. Lancet 353: 22–25. [DOI] [PubMed] [Google Scholar]
- 35.Carr R, 2001. Excreta-related infections and the role of sanitation in the control of transmission. In: Fewtrall L and Bartram J, eds. Water Quality Guidelines, Standards and Health. London, United Kingdom: IWA Publishing. [Google Scholar]
- 36.Nakagiri A, Niwagaba CB, Nyenje PM, Kulabako RN, Tumuhairwe JB, Kansiime F, 2016. Are pit latrines in urban areas of Sub-Saharan Africa performing? A review of usage, filling, insects and odour nuisances. BMC Public Health 16: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagiri A, Kulabako R, Nyenje P, Tumuhairwe J, Niwagaba C, Kansiime F, 2015. Performance of pit latrines in urban poor areas: a case of Kampala, Uganda. Habitat Int 49: 529–537. [Google Scholar]
- 38.Ngure FM, Reid BM, Humphrey JH, Mbuya MN, Pelto G, Stoltzfus RJ, 2014. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Ann N Y Acad Sci 1308: 118–128. [DOI] [PubMed] [Google Scholar]
- 39.Harris M, Alzua ML, Osbert N, Pickering A, 2017. Community-level sanitation coverage more strongly associated with child growth and household drinking water quality than access to a private toilet in rural Mali. Environ Sci Technol 51: 7219–7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuller JA, Eisenberg JN, 2016. Herd protection from drinking water, sanitation, and hygiene interventions. Am J Trop Med Hyg 95: 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlton EJ, Eisenberg JN, Goldstick J, Cevallos W, Trostle J, Levy K, 2014. Heavy rainfall events and diarrhea incidence: the role of social and environmental factors. Am J Epidemiol 179: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bandyopadhyaya S, Kanji S, Wang L, 2012. The impact of rainfall and temperature variation on diarrheal prevalence in Sub-Saharan Africa. Appl Geogr 33: 63–72. [Google Scholar]
- 43.Crocker J, Saywell D, Bartram J, 2017. Sustainability of community-led total sanitation outcomes: evidence from Ethiopia and Ghana. Int J Hyg Environ Health 220: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orgill-Meyer Jet al. , 2019. Long-term impact of a community-led sanitation campaign in India, 2005–2016. Bull World Health Organ 97: 523–533A. [DOI] [PMC free article] [PubMed] [Google Scholar]