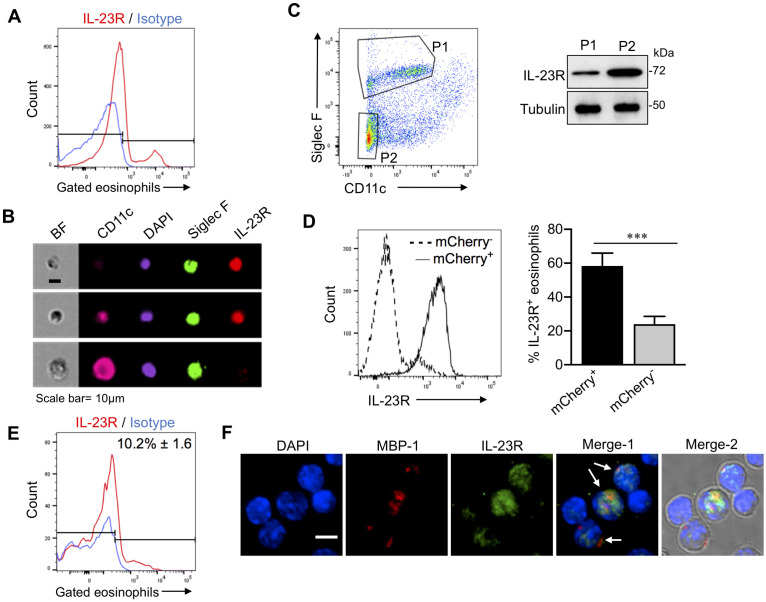
Fig 4. Eosinophil IL-23R expression from mice with acute and allergic pulmonary aspergillosis.
(A) Flow cytometry (allergy model). Allergic aspergillosis was induced in C57BL/6 mice as shown in Fig 1A. Single cells were prepared from harvested lungs and stained for CD45, CD11c, Siglec F and IL-23R. Live cells in the eosinophil gate were then analyzed by flow cytometry. A representative histogram is shown comparing IL-23R expression on eosinophils with an isotype control. The means ± SE (n = 5 mice) of IL-23R-positive eosinophils are shown next to the histogram. (B) Imaging flow cytometry (allergy model). As in Fig 4A except cells were analyzed by imagining flow cytometry. Shown are representative output images (original magnification 20X). The top two panels demonstrate representative IL-23R+ eosinophils, one of which is CD11c- and the other CD11cint. The lower panel shows a presumed alveolar macrophage (AMφ), distinguished from an eosinophil based on its larger size, high levels of CD11c expression and nuclear morphology. BF; Bright field. Cells shown are representative of >100 analyzed cells. Scale bar = 10μm. (C) Immunoblotting (allergy model). Allergic aspergillosis was induced in C57BL/6 mice as in Fig 1A, following which lungs were harvested and single cell preparations were made. The left figure shows the sorting gates of the two different cell populations used for immunoblotting of IL-23R. P1: eosinophils, P2: SigF-CD11c- cells. Whole cell extracts were made, resolved by SDS-Page and immunoblotted with primary antibodies for IL-23R and α-tubulin. The immunoblot is representative of 2 independent experiments. Tubulin was used as a loading control. (D) Flow cytometry (allergy model, mCherry reporter mice). Allergic aspergillosis was induced in RORγt-mCherry reporter mice as described in Fig 1A. After harvesting lungs, single cell lung preparations were made. By flow cytometry, mCherry negative (mCherry-) and mCherry positive (mCherry+) lung eosinophils were analyzed for the presence of IL-23R. Left panel: Representative histograms of IL-23R expression in the mCherry- and mCherry+ eosinophils. Right panel: Bar graphs showing the mean ± SEM percent IL-23R+ eosinophils in mCherry+ and mCherry- eosinophils from two independent experiments, each of which had 4–5 mice (p <0.001 comparing the two groups). (E) Flow cytometry (acute infection model). C57/BL6 mice were infected with 5x107Af293 conidia. Mice were euthanized 48 hours post-infection, single cell suspensions were made from harvested lungs, and analyzed for IL-23R expression as in Fig 4A. Histograms show the shift in IL-23R signal intensity in gated eosinophils. A representative histogram is shown comparing IL-23R expression on eosinophils with an isotype control. The means ± SE (n = 5–6 mice) of IL-23R-positive eosinophils are shown next to the histogram. (F) Confocal microscopy (acute infection model). As in Fig 4D except cells were analyzed by confocal microscopy. Merge-1: DAPI + MBP (red) + IL-23R (green). Arrows point to eosinophils identified based on MBP staining. Merge-2: DAPI (blue) + MBP (red) + IL-23R (green) + brightfield. Scale bar = 10 μm.