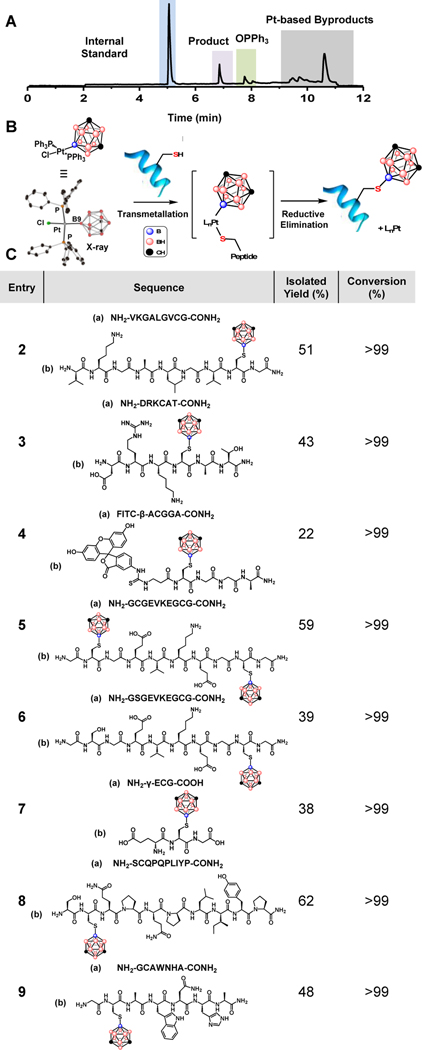
Figure 2.
A: Representative LC trace collected after 1 (1.2 equiv) and H2N-VKGALGVCG-CONH2 (5 mM) were allowed to react for 1.5 h at 25 °C in the presence of Tris•HCl buffer (30 mM) in dimethylformamide (DMF). Internal standard was produced through alkylation of H2N-VKGALGVCG-CONH2 (see SI section I). B: Proposed reaction scheme between 1 and cysteine-containing peptide C: Peptide substrate scope with isolated yields (%) and conversion (%).