Abstract
BACKGROUND:
Coronavirus disease-19 (COVID-19), resulting from infection with SARS-CoV-2, spans a wide spectrum of illness. In severely ill patients, highly elevated serum levels of certain cytokines and considerable cytolytic T cell infiltrates in the lungs have been observed. These same patients may bear low to negligible viral burdens suggesting that an overactive immune response, often termed cytokine storm, contributes to the severity of COVID-19. We report the safety and efficacy of baricitinib combined with remdesivir and dexamethasone in 45 hospitalized patients with COVID-19 pneumonia at a tertiary academic medical center.
METHODS:
Retrospective review of 45 patients hospitalized with COVID-19 pneumonia. Patients received 7-day course of baricitinib, 5-day course of remdesivir and 10-day course of dexamethasone. Clinical status and biomarkers were obtained daily. Outcomes assessed include mortality, duration of hospitalization, presence of shock, need for supplemental oxygen, need for non-invasive ventilation, need for mechanical ventilation and development of thrombosis.
RESULTS:
Obesity and multiple medical comorbidities were associated with hospitalization in the setting of COVID-19. Treated patients demonstrated rapid declines of C-reactive protein (CRP), ferritin and D-dimer with gradual improvement in hemoglobin, platelet counts and clinical status. Only 2 of 45 (4.4%) treated patients required mechanical ventilation after initiating treatment and there were six deaths (13.3%).Only 2 of 45 (4.4%) treated patients required mechanical ventilation after initiating treatment. There were six deaths (13.3%) and these were associated with lower BMI.
CONCLUSIONS:
These findings support the utility of immunosuppression via JAK inhibition in moderate to severe COVID-19 pneumonia.
Keywords: Coronavirus disease-19 (COVID-19), JAK inhibitor, baricitinib, cytokine storm, critical care
Background:
Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) has infected over 164 million people worldwide, resulting in over 3.39 million deaths to date. Despite the benefits of remdesivir and dexamethasone (1), substantial morbidity and mortality due to Coronavirus Disease 2019 (COVID-19) remain. Studies of various viral infections in animal models have suggested that an excessive immune response can promote hyperinflammation and multi-organ immune-mediated pathology (2). This is consistent with findings of elevated levels of several cytokines in severe cases of other coronavirus infections including Severe Acute Respiratory Syndrome coronavirus 1 (SARS-CoV-1) and Middle East Respiratory Syndrome coronavirus (MERS-CoV) (3, 4), as well as murine models of the 1918 influenza (5).
SARS-CoV-2 suppresses the initial type I Interferon (IFN-I) response that is critical for early control of viral infections (2, 6). This allows the virus to escape early immune suppression and to replicate more extensively. When the adaptive immune response is later engaged, profound activation of viral-specific cytolytic T cells occurs in response to the high viral burden (7). The massive expansion of cytolytic T cells causes considerable tissue damage to virally infected cells and potential damage to other uninfected tissues as innocent bystanders (8–10). This is associated with an elevation of several serum cytokines, such as interferon (IFN)-γ, interleukin (IL)-2, IL-6, IL-10, and the α-chain of the IL-2 receptor (CD25) (11, 12), a phenomenon termed cytokine release syndrome. A multi-center, retrospective study of 150 patients with severe COVID-19 showed a strong association between elevated ferritin and IL-6 levels and adverse clinical outcomes (13). These observations have collectively suggested that immunosuppressive therapy might mitigate the severity of COVID-19 infections by reducing cytokine release syndrome as well as inhibiting an overactive cytolytic T cell response.
Various anti-cytokine therapies have been considered for the cytokine release syndrome observed in COVID-19. These include tocilizumab, an IL-6 receptor(R) inhibitor (7, 14), anakinra, an IL-1R antagonist (15), and inhibitors of the Janus kinase (JAK) pathways (16–19). Baricitinib, an inhibitor of JAK1/2, was approved for treatment of rheumatoid arthritis in 2018 (20, 21). It inhibits the intracellular cytokine signaling pathways known to be active in severe COVID-19, and reduces levels of IL-2, IL-6, IL-10, IFN-γ, and granulocyte–macrophage colony-stimulating factor (GM-CSF), some of which are important to the cytolytic T cell response. In February 2020 baricitinib was proposed as a potential treatment for COVID-19 based on artificial intelligence algorithms (22). The authors hypothesized that baricitinib could directly mitigate the inflammatory response triggered by SARS-CoV-2 infection, and was also identified as a numb-associated kinase (NAK) inhibitor with high-affinity for AP2-associated protein kinase 1 (AAK1). AAK1 was previously described as a crucial regulator of clathrin-mediated endocytosis of coronaviruses and other viruses (23). This suggested baricitinib could have direct antiviral effects by preventing virus entry into target cells. This additional mechanism could be complementary to the potential benefits of inhibiting the cytokine storm associated with severe COVID-19.
In three case series of patients with COVID-19, baricitinib use was associated with improved oxygenation and reduction of certain inflammatory markers (16–19). The placebo-controlled ACTT-2 study found that baricitinib for 14 days plus remdesivir improved recovery times by one day and reduced 28-day mortality from 7.8–5.1% (16). We report our assessment of the safety and efficacy of baricitinib for approximately 7 days following initiation of treatment with combined remdesivir and dexamethasone in 45 inpatients with moderate to severe COVID-19 pneumonia at a tertiary academic medical center.
Methods:
Patients.
Between July 10, 2020, and February 8, 2021, COVID-19-positive inpatients were considered for treatment with baricitinib 4 mg daily (if glomerular filtration rate (GFR) > 60 mL/min), 2 mg daily (if GFR 30–59 mL/min), or 1 mg daily (if GFR 15–29 mL/min) for up to 7 days or hospital discharge, whichever was shorter. Inclusion criteria included a positive SARS-CoV-2 polymerase chain reaction (PCR) test, and one or more of the following: a chest X-ray demonstrating infiltrates compatible with COVID-19 infection, oxygen saturation < 94% on room air, or the need for supplemental oxygen and/or mechanical ventilation. Exclusion criteria included GFR < 15 mL/min or receiving dialysis, active infection with tuberculosis, absolute lymphocyte count < 200 cells/mL, absolute neutrophil count < 500 cells/mL, and suspected drug-induced liver injury. Patients meeting inclusion criteria also received remdesivir 200 mg intravenous (IV) for the first dose followed by 100 mg IV daily for up to 4 days or hospital discharge, whichever came first. Patients also received dexamethasone 6 mg IV daily for 10 days or until hospital discharge, whichever was shorter. Forty-five patients met the criteria and were treated with baricitinib.
Variables.
Daily laboratory results were recorded from routine patient care including hemoglobin (g/dL), white blood cell count (K/cm2), lymphocyte count (K/cm2), platelet count (K/cm2), C-reactive protein (CRP) (g/dL), ferritin (ng/ml), D-dimer (ng/ml), creatinine (g/dL), alanine transaminase (ALT) (U/L) and aspartate transaminase (AST) (U/L). Clinical illness severity was recorded at the time of admission and followed daily using both the National Early Warning Score-2 (NEWS-2 score) and an 8-point ordinal scale which had been used in the adaptive COVID-19 Treatment Trial 1 and 2 (“ACTT-CS” score) (16).
Outcomes.
Duration of hospitalization, presence of shock, and development of thrombosis during hospitalization were recorded. The presence and duration of supplemental oxygen requirement, noninvasive ventilation, and mechanical ventilation were followed. Patient discharge destination and mortality were also recorded.
Statistics.
The start date of baricitinib therapy for each patient was arbitrarily set as day 0. All observations were divided into early and late periods. The early treatment period ran from the date of the first available laboratory result (generally day − 3) through the day of the first baricitinib dose (Day 0). The late period ran from the day after starting baricitinib until the last available laboratory result. All statistical analyses were performed in Stata 16.1 (StataCorp, LLC, College Station, Texas, USA).
Results:
Patient demographics
Of 45 COVID-19 inpatients treated with baricitinib plus dexamethasone and remdesivir, the mean age was 69.5 years (SD +/− 16.7) and 53.3% were female. The average patient was obese (BMI 31), and 84.4% of patients had two or more co-existing medical conditions. The most common medical conditions were hypertension (19%), type 2 diabetes mellitus (15%), hyperlipidemia (11%), autoimmune disease (8%), and obstructive airway disease (8%) (Table 1).
Table 1. Baseline patient demographics and characteristics of COVID-19 positive inpatients treated with baricitinib / remdesivir / dexamethasone.
Plus–minus(+/−) values are standard deviation. Patient race and ethnicity are self reported by patient. Body mass index is calculated by weight (kilograms) divided by square of height (meters). Percentages may not total 100 because of rounding.
| Baseline patient demographics and characteristics | |||
|---|---|---|---|
| Characteristic | All Patients | ||
| Age - years | Mean age | 69.5 | +/−16.7 |
| Gender - no. | Male | 21 | (46.7) |
| Female | 24 | (53.3) | |
| Ethnicity – no. (%) | Not Hispanic, Latino/a, or Spanish origin | 44 | (97.8) |
| Hispanic or Latino | 1 | (2.2) | |
| Race – no. (%) | White | 36 | (80.0) |
| Asian | 4 | (8.9) | |
| African-American or Black | 2 | (4.4) | |
| Multiracial | 1 | (2.2) | |
| Declined | 2 | (4.4) | |
| Past Medical History - no. (%) | Co-existing conditions: None | 1 | (2.2) |
| Co-existing conditions: One | 6 | (13.3) | |
| Co-existing conditions: Two or more | 38 | (84.4) | |
| Average Body Mass Index | 31 | ||
| Body Mass Index ≥ 30 - no. (%) | 22 | (48.9) | |
| Body Mass Index ≥ 40 - no. (%) | 5 | (11.1) | |
| Hypertension | 19 | (42.2) | |
| Type 2 Diabetes Mellitus | 15 | (33.3) | |
| Hyperlipidemia | 11 | (24.4) | |
| Autoimmune disease (Rheumatoid arthritis, Psoriatic arthritis, Sjogren’s Syndrome, Raynaud’s phenomena, Giant cell arteritis, Ulcerative colitis, Pemphigus vulgaris) | 8 | (17.8) | |
| Obstructive airway disease (asthma, COPD, bronchiectasis and reactive airway disease) | 8 | (17.8) | |
| Obstructive Sleep Apnea | 7 | (15.6) | |
| Coronary Artery Disease | 6 | (13.3) | |
| Atrial Fibrillation | 6 | (13.3) | |
| Heart failure (including: HFpEF, HFrEF or unspecified) | 4 | (8.9) | |
| Chronic Kidney Disease | 4 | (8.9) | |
| History of Malignancy | 3 | (6.7) | |
| Aortic stenosis | 3 | (6.7) | |
| Osteoarthritis | 3 | (6.7) | |
| Pulmonary Hypertension | 2 | (4.4) | |
| Average duration of symptoms prior to presentation – days | 6 | ||
| Average severity of illness at presentation | NEWS2 score | 5 | |
| ACTT-CS Score | 4 | ||
| Dose of Baricitinib - no. (%) | Baricitinib 4mg daily or 2mg twice daily | 34 | (75.6) |
| Less than Baricitinib 4mg daily or 2mg twice daily | 11 | (24.4) | |
| Duration of Baricitinib - no. (%) | Less than 7-days – no. | 22 | (48.9) |
| 7-days – no. | 20 | (44.4) | |
| More than 7-days – no. | 3 | (6.7) | |
| Average duration of baricitinib - (days) | 6.0 | ||
The mean duration of baricitinib treatment was 6 days, and 44% of patients completed a 7-day course of baricitinib (Table 1). Twenty-two patients did not complete the full 7-day course of baricitinib: 9 patients were discharged before completing the treatment course, medication was discontinued in 8 inpatients in the setting of patient stabilization, 6 patients died, and 1 patient left against medical advice (Tables 1). Three patients completed longer courses of baricitinib based on clinical judgement: one patient completed an 11-day course, one patient completed 12 days, and one patient completed two 7-day courses separated by two days for a total of 14 days. 93.3% of patients started baricitinib within 24-hours of receiving dexamethasone and remdesivir.
At the time of initial presentation, patients had symptoms for an average of 6 days and had a mean aggregate NEWS-2 score of 5 (medium clinical risk requiring an urgent response). Seventy-five percent of patients had bilateral pneumonia on initial chest X-ray. On admission, 60% of patients required supplemental oxygen, 22% required non-invasive ventilation, and 4% required invasive mechanical ventilation (Table 2).
Table 2. Outcomes among of COVID-19 positive inpatients treated with baricitinib / remdesivir / dexamethasone.
Percentages may not total 100 because of rounding.
| Patient Outcomes | |||
|---|---|---|---|
| All Patients | |||
| Primary Outcomes: | |||
| Mortality over first 7-days following admission – no. (%) | 2 | (4.4) | |
| Mortality over entire hospitalization? – no. (%) | 6 | (13.3) | |
| Secondary Outcomes: | |||
| Diagnosis of hemodynamic shock during hospitalization – no. (%) | 4 | (8.9) | |
| Thrombosis during hospitalization – no. (%) | - | ||
| Need for vasopressor support during hospitalization – no. (%) | 4 | (8.9) | |
| Average duration of vasopressor support care during hospitalization – days | 1.5 | ||
| New oxygen requirement during hospitalization – no. (%) | 45 | (100.0) | |
| Average duration of new oxygen requirement (days) | 7.9 | ||
| Need for non-invasive ventilation – no. (%) | 19 | (42.2) | |
| Average duration of non-invasive ventilation (days) | 5.1 | ||
| Need for mechanical ventilation during hospitalization – no. (%) | 4 | (8.9) | |
| Average duration of mechanical ventilation during hospitalization (days) | 3.75 | ||
| Duration of hospitalization (days) | 11 | ||
| Estimated duration of illness (days) | 15 | ||
| Discharge status - | |||
| Home or self care – no. (%) | 26 | (57.8) | |
| Home with home health services – no. (%) | 5 | (11.1) | |
| Subacute rehab – no. (%) | 4 | (8.9) | |
| Skilled nursing facility – no. (%) | 2 | (4.4) | |
| Transfer to another facility – no. (%) | 2 | (4.4) | |
| Deceased – no. (%) | 6 | (13.3) | |
Laboratory and clinical variables:
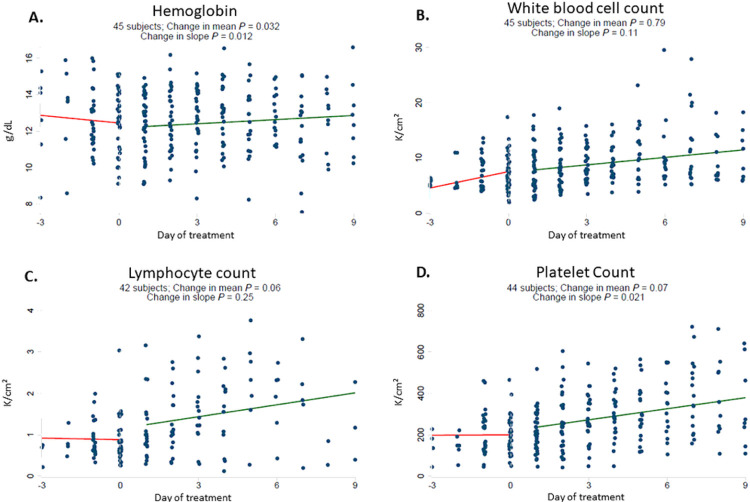
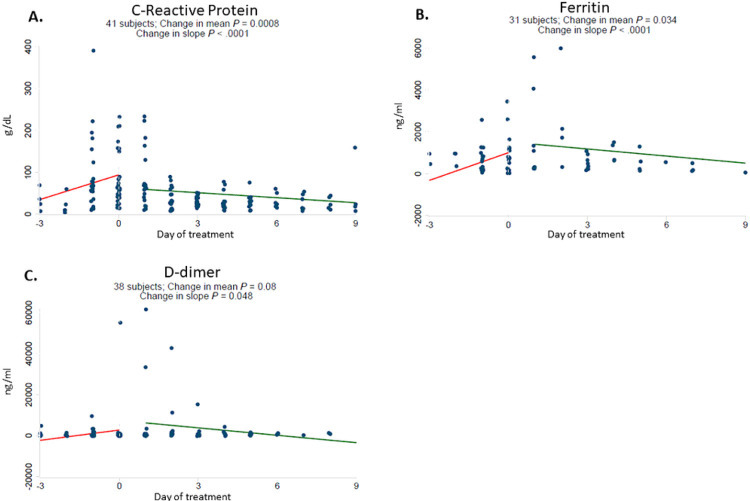
In response to starting baricitinib, dexamethasone and remdesivir therapy, hemoglobin reversed its downward trend and increased (Fig. 1A). Platelet counts significantly increased and there was no significant change in white blood cell count (Fig. 1B). Rising levels of CRP, D-dimer, and ferritin also reversed rapidly and significantly after starting baricitinib (Fig. 2). There was no significant change in creatinine or ALT, whereas AST declined significantly following therapy (Supplemental Fig. 1).
Figure 1.
Baricitinib plus dexamethasone and remdesivir therapy reverses downward trajectory of hemoglobin and increase platelet count. Daily laboratory results were recorded from routine patient care including hemoglobin (g/dL) [A], white blood cell count (K/cm2) [B], lymphocyte count (K/cm2) [C] and platelet count (K/cm2) [D]. The start date of baricitinib, dexamethasone and remdesivir therapy for each patient was arbitrarily set as day 0. All observations were divided into early (up to day −3) and late periods (up to day +9). Linear regressions were perform for each variable and a difference in slope tested between before treatment (day −3 to day 0) vs. after treatment (day +1 to day +9).
Figure 2.
Baricitinib plus dexamethasone and remdesivir therapy reverses upward trajectory of C-reactive protein, Ferritin and D-dimer. Daily laboratory results were recorded from routine patient care including C-reactive protein (g/dL) [A], ferritin (ng/ml) [B] and D-dimer (ng/ml) [C]. The start date of baricitinib, dexamethasone and remdesivir therapy for each patient was arbitrarily set as day 0. All observations were divided into early (up to day −3) and late periods (up to day +9). Linear regressions were perform for each variable and a difference in slope tested between before treatment (day −3 to day 0) vs. after treatment (day +1 to day +9).
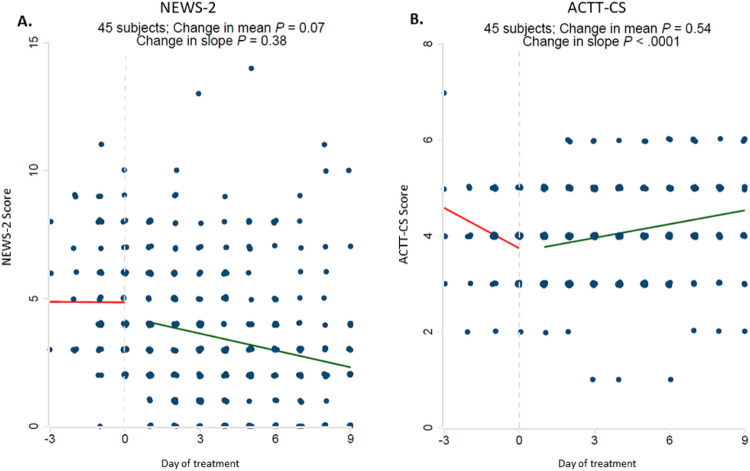
There was also a significant improvement in ACTT-CS clinical status scores in response to treatment (Fig. 3). NEWS-2 scores also improved with treatment, although this did not achieve statistical significance.
Figure 3.
Significant improvement in ACTT clinical status scores (ACTT-CS) in response to Baricitinib plus dexamethasone and remdesivir therapy. Clinical illness severity was recorded at the time of admission and followed daily using the National Early Warning Score-2 (NEWS-2 score) [A] and an 8-point ordinal scale which had been used in the adaptive COVID-19 Treatment Trial 1 and 2 (“ACTT-CS” score) [B]. There was no statistical significant change in NEWS-2 scores. The start date of baricitinib, dexamethasone and remdesivir therapy for each patient was arbitrarily set as day 0. All observations were divided into early (up to day −3) and late periods (up to day +9). Linear regressions were performed for each variable and a difference in slope tested between before treatment (day −3 to day 0) vs. after treatment (day +1 to day +9).
Patient outcomes
The average duration of hospitalization was 11 days. Four patients (8.9%) experienced hemodynamic shock. All patients required supplemental oxygen at some point during their admission and 19 required non-invasive ventilation (42.2%) with an average duration of 5 days. Four patients required mechanical ventilation (8.9%) during hospitalization, with an average duration of 3.75 days. However, only two patients required mechanical ventilation following initiation of baricitinib (one patient was transferred from an outside hospital and a second patient was intubated at time of initial presentation prior to starting baricitinib). 68.9% of patients were discharged home with self-care or home health services. 13.3% of patients were discharged to subacute rehabilitation or a skilled nursing facility. There were 6 deaths (13.3%) (Table 2.)
Subgroup analysis
Of the 6 treated patients who died, 2 died within the first 7 days of hospitalization. Deaths occurred in patients who were significantly older (mean age 84.5 [SD +/− 7.4]), had a lower BMI (mean 24), and had more severe illness at initial presentation (average aggregate NEWS-2 score = 7, High clinic risk suggestive of urgent response with need for continuous monitoring) (Table 3.). Two of the deaths were complicated by the presence of sub-massive pulmonary emboli on admission, prior to initiating any therapy (Supplemental Table 1.).
Table 3. Baseline patient demographics and characteristics of COVID-19 positive inpatients treated with baricitinib / remdesivir / dexamethasone who did died during hospitalization. Plus–minus(+/−) values are standard deviation.
Patient race and ethnicity are self-reported by patient. Body mass index is calculated by weight (kilograms) divided by square of height (meters). Percentages may not total 100 because of rounding.
| Deceased Patients | |||
|---|---|---|---|
| Age - years | Mean age (year) | 84.5 | SD +/− 7.4 |
| Gender - no. | Male (n) | 4 | (66.7) |
| Female (n) | 2 | (33.3) | |
| Ethnicity – no. (%) | Not Hispanic, Latino/a, or Spanish origin (n) | 6 | (100.0) |
| Hispanic or Latino (n) | 0 | ||
| Race – no. (%) | White (n) | 5 | (83.3) |
| Asian (n) | 0 | - | |
| African-American or Black (n) | 1 | (16.7) | |
| Multiracial (n) | 0 | - | |
| Declined (n) | 0 | - | |
| Past Medical History - no. (%) | Co-existing conditions: None | 0 | - |
| Co-existing conditions: One | 0 | - | |
| Co-existing conditions: More than 2 | 6 | 100.0 | |
| Average BMI | 24 | ||
| Obesity - BMI: >30 | 1 | (16.7) | |
| Morbid Obesity - BMI: > 40 | 0 | - | |
| Obstructive Sleep apnea | 1 | (16.7) | |
| Coronary artery disease | 1 | (16.7) | |
| Hypertension | 4 | (66.7) | |
| Type 2 Diabetes Mellitus | 2 | (33.3) | |
| Hyperlipidemia | 2 | (33.3) | |
| Heart failure (HFpEF, HFrEF or unspecified) | 1 | (16.7) | |
| Chronic Kidney Disease | 1 | (16.7) | |
| Atrial Fibrillation | 3 | (50.0) | |
| History of Malignancy | 1 | (16.7) | |
| Pulmonary hypertension | 2 | (33.3) | |
| Aortic stenosis | 3 | (50.0) | |
| Osteoarthritis | 1 | (16.7) | |
| Autoimmune disease (Rheumatoid arthritis, Psoriatic arthritis, Sjogren’s Syndrome, Raynaud’s phenomena, GCA, UC, Pemphigus vulgaris) | 3 | (50.0) | |
| Obstructive airway disease (asthma, chronic obstructive pulmonary disease, bronchiectasis and reactive airway disease) | 2 | (33.3) | |
| Average duration of symptoms prior to presentation - days | 3 | (52.8) | |
| Average severity of illness at presentation | NEWS2 score | 7 | |
| ACTT-2 Clinical Status Score | 4 | ||
| Bilateral Pneumonia on imaging at presentation - no. (%) | 4 | (66.7) |
Discussion:
The current findings support the use of immunosuppressive therapy in moderate to severe COVID-19 using a combination of corticosteroids and a JAK1/2 inhibitor. All patients in this study also received remdesivir and dexamethasone in addition to baricitinib, so it is not possible to assess the individual contribution to recovery of each medication. This study spans a 6-month period during which SARS-CoV2 variants emerged in Vermont, USA.
Four patients required mechanical ventilation (8.9%) during their hospitalization. Of these, two received ventilator support prior to starting baricitinib therapy, and one of these was extubated shortly after initiating baricitinib. This contrasts with the average higher mechanical ventilation rate of 14.5% from a meta-analysis of 12,437 COVID-19 ICU admissions (24), and was considerably lower than our own experience in the early phases of the pandemic. The patient population in the current study was equally distributed between males and females, in contrast to many other studies. In addition, half were age 70 or older, which makes the favorable outcome all the more remarkable. Finally, there were six deaths (13.3%) among the 45 patients. Two of these, however, were complicated by sub-massive pulmonary emboli present at the time of admission, prior to initiation of baricitinib.
This study sought to assess the efficacy and safety of 7 days of baricitinib treatment, whereas other recent trials in COVID-19 patients treated for 14 days (16–19). The decision for a shorter treatment period was made out of a desire to balance the suppression of inflammation that might result in tissue damage with an avoidance of prolonged immunosuppression that might delay viral clearance or promote secondary infections. Delays in viral clearance have in fact been observed in other immunocompromised patients, resulting in the emergence of viral variants (25). Additionally, given the known risk of JAK inhibitor-induced thrombosis, in the context of the recognized coagulopathy risk in COVID-19, a shorter treatment course may be favorable and sufficient for the duration of cytokine release syndrome in these patients.
Half of the patients with moderate to severe COVID-19 had a BMI greater than 30. This is considerably higher than the 23.2% obesity prevalence for the general population in Vermont (https://www.cdc.gov/obesity/stateprograms/fundedstates/pdf/vermont-state-profile.pdf). Obesity is a known risk factor for severe COVID-19 infection (26). Obesity is also associated with a baseline inflammatory state (27). Adipose tissue supports the development of tissue resident T lymphocytes that upregulate gene expression for several inflammatory cytokines as well as for cytolytic activity, and express high levels of the checkpoint blocker programmed cell death protein-1 (PD-1) (28). A very similar phenotype of T cells is observed in bronchiolar lavage fluid of COVID-19 patients (29). Additionally our retrospective review echoes prior work by the Center for Disease Control that older age is associated with increased risk of hospitalization and poorer prognosis with COVID-19 infection.
No adverse effects were noted from use of baricitinib. In particular, there were no secondary infections. Despite concern for increased thrombotic risk with baricitinib, we did not observe clinical evidence of new clots during the brief course of baricitinib treatment, although two patients demonstrated significant clots on admission prior to initiation of baricitinib.
SARS-CoV-2 is known to suppress the initial IFN-I response, likely through the interaction of particular viral proteins with molecules of the IFN-I signaling pathway (30, 31). This allows the virus to rapidly replicate during the early stages of infection. The delayed immune response can then become hyperactive and result in considerable cell death of surrounding tissues. This could include tissues that are not known to support SARS-CoV-2 replication, such as liver inflammation observed in some cases of severe COVID-19 (32). The subsequent release of host RNA and DNA from damaged tissues can strongly activate, respectively, the retinoic acid-inducible gene 1 (RIG-I) and cyclic GMP-AMP synthase (cGAS) nucleic acid sensing pathways, leading to an augmented IFN-I response and persistent inflammation even in the absence of virus. This is consistent with studies showing that death of lung epithelium is due in some instances more to the immune response than to viral-mediated lysis (33). Emerging evidence in animal models of SARS and MERS has revealed that the initial IFN-I response has beneficial effects in the early phases of disease, but may become damaging in the latter phases (34).
Severe COVID-19 has close parallels with other seemingly unrelated syndromes that might collectively be classified as hyperinflammatory disorders. Chimeric antigen receptor T (CAR-T) cell therapy exposes patients to a large number of T cells that become activated upon contact with targeted tumor cells, often resulting in a highly inflammatory cytokine release syndrome that can include hypercoagulation and even acute respiratory distress syndrome (ARDS) (35–37). Toxic shock syndrome is a multiorgan inflammatory syndrome (38) in which tampons infected with Staphylococcus release an enterotoxin that acts as a superantigen by binding both the MHC class II molecule and the β-chain of several T cell receptors (39). This activates a significant portion of the T cell repertoire, similar to CAR-T therapy, resulting in injury to many organs including skin, liver, and lung, and can also be associated with coagulopathy and ARDS (38). Consistent with the view of hyperactivation of T cells in these disorders, individuals with HIV and low T cell counts have been noted to have less severe COVID-19 (40).
An additional parallel can be made between severe COVID-19 and hemophagocytic lymphohistiocytosis (HLH). HLH is a severe inflammatory syndrome characterized by fever, hepatitis, spleen and lymph node enlargement, and pancytopenia (41, 42). It is often observed secondary to certain viral infections as well as autoimmune syndromes such as juvenile inflammatory arthritis (41). An additional laboratory characteristic is elevated ferritin, which we observed in our severe COVID-19 cases. HLH is likely the result of strong T cell activation producing cytokines that activate macrophages to become highly phagocytic (41, 42). Consequently, anti-cytokine therapy has also been used to treat HLH, including IL-1 blockade as well as JAK inhibitors.
In other case series of patients with COVID-19, baricitinib treatment was associated with both an improvement in oxygenation and a reduction in select inflammatory markers (16–19). The largest of these, the ACTT-2 Study Group, randomized 1033 patients to receive remdesivir and either baricitinib for up to 14 days (515) or placebo control (518). Patients receiving baricitinib had a median time to recovery of 7 days compared to 8 days for the control group, and a 30% higher odds of improvement in clinical status at day 15. Patients receiving high-flow oxygen or noninvasive ventilation at enrollment had a time to recovery of 10 days with combination treatment and 18 days with control. The 28-day mortality was 5.1% in the combination group and 7.8% in the control group.
Limitations of the current study include its retrospective nature, the study site was a single center, and the study did not allow for comparison with untreated controls.
Conclusions:
We report a retrospective review of hospitalized COVID-19 patients treated with a short course of baricitinib in combination with dexamethasone and remdesivir. Consistent with previous literature we report that an elevated BMI and multiple medical comorbidities are risk factors associated with hospitalization following COVID-19 infection. Additionally a higher age, ≥ 2 co-existing medical conditions, and higher illness severity at time of presentation was associated with a poorer prognosis. Baricitinib use was associated with increase in hemoglobin and platelet count, decrease in inflammatory markers (D-dimer, Ferritin and CRP), and improvement in clinical status (specifically the ACTT-CS). The mean duration of baricitinib use was 6 days, which in light of the observed improvements in clinical status and inflammatory markers, may suggest that a shorter course could be beneficial while reducing the risk of more prolonged immunosuppression and thrombosis. No adverse effects were noted from use of baricitinib. In particular, there were no secondary infections or thrombosis following initiation of baricitinib. The current findings support the use of immunosuppressive therapy in moderate to severe COVID-19 using a combination of steroids and a JAK inhibitor.
Supplementary Material
Funding:
This work was supported by National Institutes of Health. Grant number GM118228 (RCB).
List Of Abbreviations
- AAK1
AP2-associated protein kinase 1
- ACTT-CS
Adaptive COVID-19 Treatment Trial - Clinical status score
- ARDS
Acute respiratory distress syndrome
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- CAR-T
Chimeric antigen receptor T
- cGAS
cyclic GMP-AMP synthase
- COVID-19
Coronavirus Disease 2019
- COPD
Chronic obstructive pulmonary disease
- Cr
Creatinine
- GFR
Glomerular filtration rate
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- HLH
Hemophagocytic lymphohistiocytosis
- HFrEF
Heart failure reduced ejection fraction
- HFpEF
Heart failure preserved ejection fraction
- IFN-I
Type I Interferon
- IL
Interleukin
- IV
intravenous
- JAK
Janus kinase
- MERS-CoV
Middle East Respiratory Syndrome coronavirus
- NAK
Numb-associated kinase
- NEWS-2
National Early Warning Score-2
- PCR
Polymerase chain reaction
- PD-1
Programmed cell death protein-1
- RIG-I
Retinoic acid-inducible gene 1
- SARS-CoV-1
Severe Acute Respiratory Syndrome coronavirus 2
- SARS-CoV-2
Severe Acute Respiratory Syndrome coronavirus 2
- SD
Standard Deviation
Footnotes
Ethics Approval and Consent to participate:
Ethics committee review and approval waived as project was retrospective and did not include patient identifiers.
Competing interests:
The authors declare that they have no competing interests.
Supplementary Files
Contributor Information
Brendan Lawless Thoms, University of Vermont Medical Center.
Jeanne Gosselin, University of Vermont Medical Center.
Bonita Libman, University of Vermont Medical Center.
Benjamin Littenberg, University of Vermont College of Medicine.
Ralph Budd, University of Vermont College of Medicine.
Availability of data:
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA. and tenOever B. R.. 2020. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181: 1036–1045 e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okabayashi T, Kariwa H, Yokota S, Iki S, Indoh T, Yokosawa N, Takashima I, Tsutsumi H, Fujii N. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Wit E, Siegers JY, Cronin JM, Weatherman S, van den Brand JM, Leijten LM, van Run P, Begeman L, van den Ham HJ, Andeweg AC, Bushmaker T, Scott DP, Saturday G, Munster VJ, Feldmann H, van Riel D. 1918 H1N1 Influenza Virus Replicates and Induces Proinflammatory Cytokine Responses in Extrarespiratory Tissues of Ferrets. J Infect Dis. 2018;217:1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen CK, Lam JY, Wong WM, Mak LF, Wang X, Chu H, Cai JP, Jin DY, To KK, Chan JF, Yuen KY. and Kok K. H.. 2020. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg Microbes Infect: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusnadi A, Ramirez-Suastegui C, Fajardo V, Chee SJ, Meckiff BJ, Simon H, Pelosi E, Seumois G, Ay F, Vijayanand P. and Ottensmeier C. H.. 2020. Severely ill COVID-19 patients display augmented functional properties in SARS-CoV-2-reactive CD8 + T cells. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meckiff BJ, Ramirez-Suastegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, Eschweiler S, Grifoni A, Pelosi E, Weiskopf D, Sette A, Ay F, Seumois G, Ottensmeier CH, Vijayanand P. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell. 2020;183:1340–53. e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, Sette A. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–501. e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangalmurti N, Hunter CA. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–4. [DOI] [PubMed] [Google Scholar]
- 13.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992–1000.e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franzetti M, Forastieri A, Borsa N, Pandolfo A, Molteni C, Borghesi L, Pontiggia S, Evasi G, Guiotto L, Erba M, Pozzetti U, Ronchetti A, Valsecchi L, Castaldo G, Longoni E, Colombo D, Soncini M, Crespi S, Maggiolini S, Guzzon D, Piconi S. IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective, Observational Study. J Immunol. 2021;206:1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel J. H., and A.-S. G. Members. 2021. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med 384: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Cane S, Batani V, Trovato R, Fiore A, Petrova V, Hofer F, Barouni RM, Musiu C, Caligola S, Pinton L, Torroni L, Polati E, Donadello K, Friso S, Pizzolo F, Iezzi M, Facciotti F, Pelicci PG, Righetti D, Bazzoni P, Rampudda M, Comel A, Mosaner W, Lunardi C, Olivieri O. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130:6409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect. 2020;81:318–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stebbing J, Sanchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, Shen JX, Sommerauer C, Tiseo G, Ghiadoni L, Virdis A, Monzani F, Rizos LR, Forfori F, Avendano Cespedes A, De Marco S, Carrozzi L, Lena F, Sanchez-Jurado PM, Lacerenza LG, Cesira N, Caldevilla Bernardo D, Perrella A, Niccoli L, Mendez LS, Matarrese D, Goletti D, Tan YJ, Monteil V, Dranitsaris G, Cantini F, Farcomeni A, Dutta S, Burley SK, Zhang H, Pistello M, Li W, Romero MM, Andres Pretel F, Simon-Talero RS, Garcia-Molina R, Kutter C, Felce JH, Nizami ZF, Miklosi AG, Penninger JM, Menichetti F, Mirazimi A, Abizanda P, Lauschke VM. 2021. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, Yakushin S, Ishii T, Emoto K, Beattie S, Arora V, Gaich C, Rooney T, Schlichting D, Macias WL, de Bono S, Tanaka Y. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
- 21.Keystone EC, Genovese MC, Schlichting DE, de la Torre I, Beattie SD, Rooney TP, Taylor PC. Safety and Efficacy of Baricitinib Through 128 Weeks in an Open-label, Longterm Extension Study in Patients with Rheumatoid Arthritis. J Rheumatol. 2018;45:14–21. [DOI] [PubMed] [Google Scholar]
- 22.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Rawling M, Savory E, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang R, Elhusseiny KM, Yeh YC, Sun WZ. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis. PLoS One. 2021;16:e0246318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, Adhikari UD, Winkler ML, Mueller AA, Hsu TY, Desjardins M, Baden LR, Chan BT, Walker BD, Lichterfeld M, Brigl M, Kwon DS, Kanjilal S, Richardson ET, Jonsson AH, Alter G, Barczak AK, Hanage WP, Yu XG, Gaiha GD, Seaman MS, Cernadas M, Li JZ. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383:2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, Alsukait RF, Alluhidan M, Alazemi N, Shekar M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr. 2013;16:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, Grossenbacher SK, Withers SS, Rebhun RB, Hartigan-O’Connor DJ, Mendez-Lagares G, Tarantal AF, Isseroff RR, Griffith TS, Schalper KA, Merleev A, Saha A, Maverakis E, Kelly K, Aljumaily R, Ibrahimi S, Mukherjee S, Machiorlatti M, Vesely SK, Longo DL, Blazar BR, Canter RJ, Murphy WJ, Monjazeb AM. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wauters E, Van Mol P, Garg AD, Jansen S, Van Herck Y, Vanderbeke L, Bassez A, Boeckx B, Malengier-Devlies B, Timmerman A, Van Brussel T, Van Buyten T, Schepers R, Heylen E, Dauwe D, Dooms C, Gunst J, Hermans G, Meersseman P, Testelmans D, Yserbyt J, Tejpar S, De Wever W, Matthys P, C collaborators, Neyts J, Wauters J, Qian J, Lambrechts D. 2021. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res 31: 272–290. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O’Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Huttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing ZZC, Zhou Y, Peng S, Shi Y, Zhang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Lyu J, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Rakesh R, Liu X, Rosenthal SB, Calviello L, Venkataramanan S, Liboy-Lugo J, Lin Y, Huang XP, Liu Y, Wankowicz SA, Bohn M, Safari M, Ugur FS, Koh C, Savar NS, Tran QD, Shengjuler D, Fletcher SJ, O’Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, Sharp PP, Wenzell NA, Kuzuoglu-Ozturk D, Wang HY, Trenker R, Young JM, Cavero DA, Hiatt J, Roth TL, Rathore U, Subramanian A, Noack J, Hubert M, Stroud RM, Frankel AD, Rosenberg OS, Verba KA, Agard DA, Ott M, Emerman M, Jura N, von Zastrow M, Verdin E, Ashworth A, Schwartz O, d’Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor SN, Fraser JS, Gross A J. D., Sali BL, Roth D, Ruggero J, Taunton T, Kortemme, Vignuzzi Beltrao. M., Garcia-Sastre A., Shokat K. M., Shoichet B. K., and Krogan N. J.. 2020. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY. Zhong N. S., and C. China Medical Treatment Expert Group for. 2020. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, Kuri-Cervantes L, Pampena MB, D’Andrea K, Manne S, Chen Z, Huang YJ, Reilly JP, Weisman AR, Ittner CAG, Kuthuru O, Dougherty J, Nzingha K, Han N, Kim J, Pattekar A, Goodwin EC, Anderson EM, Weirick ME, Gouma S, Arevalo CP, Bolton MJ, Chen F, Lacey SF, Ramage H, Cherry S, Hensley SE, Apostolidis SA, Huang AC, Vella LA, Unit UPCP, Betts MR, Meyer NJ, Wherry EJ. 2020. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channappanavar R, Fehr AR, Zheng J, Wohlford-Lenane C, Abrahante JE, Mack M, Sompallae R, McCray PB Jr, Meyerholz DK, Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M, Berg RA, Fitzgerald JC, Aplenc R, Gore L, Grupp SA. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottlieb M, Long B, Koyfman A. The Evaluation and Management of Toxic Shock Syndrome in the Emergency Department: A Review of the Literature. J Emerg Med. 2018;54:807–14. [DOI] [PubMed] [Google Scholar]
- 38.Kulhankova K, King J, Salgado-Pabon W. Staphylococcal toxic shock syndrome: superantigen-mediated enhancement of endotoxin shock and adaptive immune suppression. Immunol Res. 2014;59:182–7. [DOI] [PubMed] [Google Scholar]
- 39.Blanco JL, Ambrosioni J, Garcia F, Martinez E, Soriano A, Mallolas J, Miro JM. and C.-i. H. Investigators. 2020. COVID-19 in patients with HIV: clinical case series. Lancet HIV 7: e314–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brito-Zeron P, Bosch X, Perez-de-Lis M, Perez-Alvarez R, Fraile G, Gheitasi H, Retamozo S, Bove A, Monclus E, Escoda O, Moreno A, Lopez-Guillermo A, Khamashta MA, Ramos-Casals M. and B. S. Group. 2016. Infection is the major trigger of hemophagocytic syndrome in adult patients treated with biological therapies. Semin Arthritis Rheum 45: 391–399. [DOI] [PubMed] [Google Scholar]
- 41.Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.