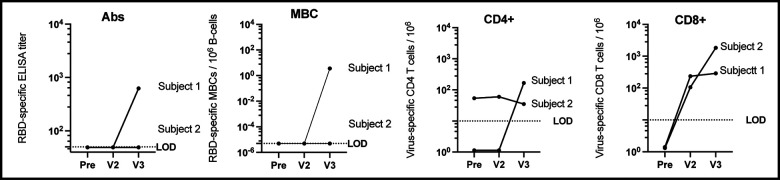
Figure 1.
Immune response to COVID-19 vaccination in CLL subjects. RBD-specific antibody (Ab) titer. Subjects without a detectable Ab titer (< 1:50 serum dilution) were assigned a value of 49. Frequency of RBD- specific MBCs per 106 CD19+ B-cells following ex vivo stimulation. Subjects who did not have a detectable response were assigned a value of 5×10−6. SARS-CoV-2 spike peptide-reactive CD4 and CD8 T-cells are defined as double positive for IFNγ and TNFα cytokine secretion. Patients who did not have a detectable T-cell response were assigned an arbitrary number between less than 2. Visit 1 (pre) blood draw was taken 21 and 40 days prior Pfizer vaccine series (2-doses). Visit 2 (V2) blood draw was taken 33 and 24 days post vaccination, and visit 3 (V3) was drawn 30 and 27 days after 3rd vaccination with J&J.