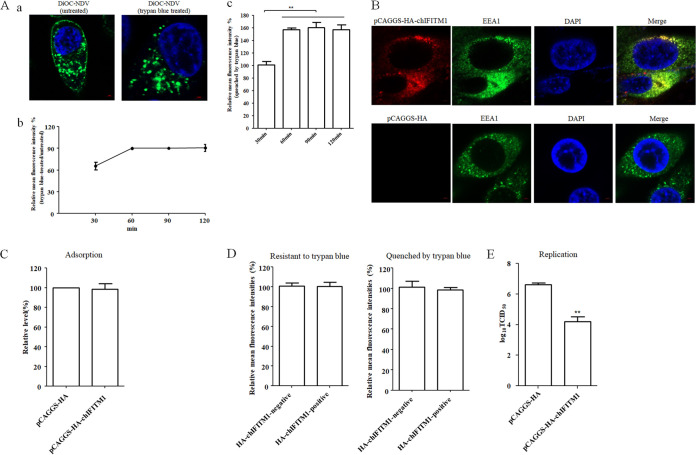
FIG 1.
NDV undergoes endocytosis and chIFITM1 located in the early endosome restricts the NDV infection. (A) NDV can be endocytosed. HD11 cells were inoculated with DiOC-labeled NDV F48E9 at 37°C for 60 min. After incubation, cells were washed with low-pH buffer to remove noninternalized viruses, and then the cells were fixed and treated with trypan blue to quench the green fluorescence of DiOC in the plasma membrane surface. The fluorescence of DiOC was examined by confocal fluorescence microscopy (a). HD11 cells were inoculated with DiOC-labeled NDV F48E9 at 37°C for different time. The cells were treated with low-pH buffer to inactivate and remove noninternalized viruses and then collected and fixed in 4% paraformaldehyde. Each cell sample was divided into two. One sample was subjected directly for the flow cytometric analysis, and the other was treated with trypan blue. The fluorescence of DiOC was examined by flow cytometry. The fluorescence intensity that was resistant (b) and quenched (c) by trypan blue at 60, 90, and 120 min was calculated relative to that of 30 min (×100%). (B) Cellular localization of chIFITM1. HD11 cells were transfected with pCAGGS-HA-chIFITM1 or pCAGGS-HA. The cells were fixed at 24 h after transfection and then subjected to indirect immunofluorescence to detect HA-tagged chIFITM1 and early endosomes marker, EEA1, using anti-HA antibody (red) and anti-EEA1 antibody (green). The nuclei were stained with DAPI (blue). (C) chIFITM1 did not inhibit NDV adsorption. HD11 cells transfected with pCAGGS-HA-chIFITM1 or pCAGGS-HA were incubated with NDV F48E9 for 1 h at 4°C to allow for viral adsorption. Viral RNA was quantified by real-time RT-PCR. (D) chIFITM1 did not inhibit NDV internalization. HD11 cells transfected with pCAGGS-HA-chIFITM1 or pCAGGS-HA were incubated with DiOC-labeled NDV F48E9 for 1 h at 4°C to allow for viral adsorption. Cells were then washed and further incubated for 1 h at 37°C to allow for internalization. After incubation, the cells were washed with low-pH buffer to remove noninternalized viruses and then collected and fixed in 4% paraformaldehyde. Each cell sample was divided into two. One sample was subjected directly for the flow cytometric analysis, and the other was treated with trypan blue. The HA-chIFITM1 was detected by using an HA tag antibody. The green fluorescence of DiOC-labeled NDV that was resistant and quenched by trypan blue in HA-chIFITM1-positive and-negative cells was measured by flow cytometry. (E) chIFITM1 reduced the replication of NDV in HD11 cells. HD11 cells were transfected with pCAGGS-HA-chIFITM1 or pCAGGS-HA. At 48 h posttransfection, cells were infected with F48E9 at an MOI of 0.1. At 18 hpi, viral titers in the culture supernatants of infected cells were determined. The bars represent means ± the SD. Data were analyzed by using the Student t test (*, P < 0.05; **, P < 0.01).