Figure 2.
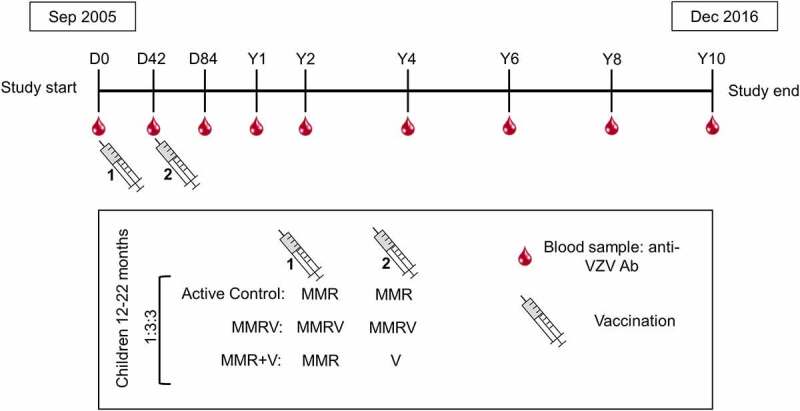
Study design
D0, day of the first vaccine dose; D42, day of the second vaccine dose (Day 42, administered six weeks after the first vaccine dose); D84, follow-up timepoint at six weeks after the second vaccine dose – Day 84; Y, year; Y1-Y10, regular blood sampling and follow-up timepoints after the second vaccine dose (after one, two, four, six, eight, and ten years); Active Control, participants receiving two doses of trivalent measles-mumps-rubella vaccine (MMR); MMRV, participants receiving two doses of the tetravalent MMR-varicella vaccine; MMR+V, participants receiving one dose of MMR and one dose of monovalent varicella vaccine; 1:3:3, randomization format of participants in the treatment groups (Active control: MMRV: MMR+V); VZV, varicella-zoster virus; Ab, antibodies.
