Figure 5.
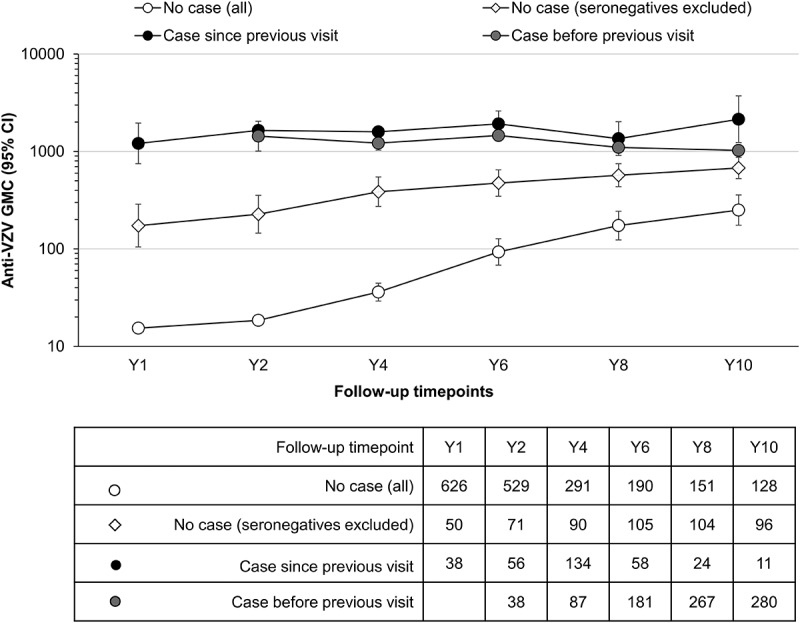
Evolution of anti-VZV antibody GMCs (lines) and the number of participants (table) in the Active Control group (per-protocol cohort for efficacy)
Case before previous visit, varicella case confirmed before blood sampling preceding the reference point; Case since previous visit, varicella case confirmed between previous and reference blood sampling; No case, no varicella case detected until the reference point (data for all of these participants and for a subset excluding the seronegative participants is shown); VZV, varicella-zoster virus; GMC, geometric mean concentration in milli-international units per mL (mIU/mL); 95% CI, 95% confidence interval; Follow-up timepoints, blood samples collected at indicated study visits after the second vaccine dose; Y1–Y10, post-vaccination blood samples obtained at year one, two, four, six, eight, and ten of follow-up.
