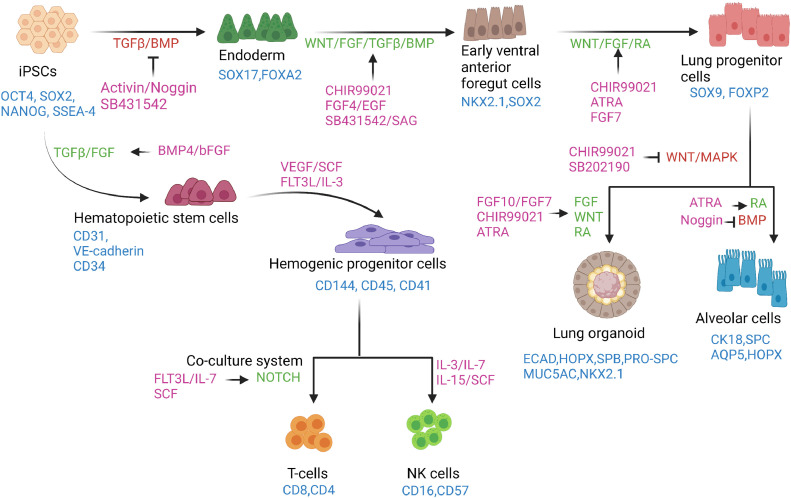
Figure 2.
Schema of directed differentiation of iPSCs to lymphocytes, alveolar cells and lung organoids in vitro. hiPSCs in a monolayer are directed to an endoderm fate by inhibiting the TGFβ and BMP pathways and subsequently to early ventral anterior foregut cells by activating WNT and FGF signaling along with the TGFβ and BMP pathways. The anterior foregut cells are directed toward a lung progenitor cell fate by stimulating the WNT, FGF and RA signaling pathways. Downstream differentiation of lung progenitors can be achieved by employing protocols that activate FGF, WNT and RA signaling or by simultaneously activating the RA pathway and inhibiting the BMP pathway to derive lung organoids and alveolar cell types, respectively. By recapitulating key stages of lymphocyte development, T and NK cells can be derived from iPSCs. First, the iPSCs are induced to hematopoietic stem cell phenotypes, which undergo further specification, giving rise to hematopoietic progenitor cells using cytokines and factors activating the TGFβ and FGF signaling pathways. The hematopoietic progenitors can be directed toward a T-cell fate using a co-culture system in the presence of molecules that primarily activate the Notch pathway. NK cell derivation from hematopoietic progenitor cells requires the presence of various cytokines. The markers of each of the cell types are shown in blue. The signaling pathways are shown in red when inhibited and in green when stimulated. Small molecules and recombinant proteins used for directed differentiation processes are shown in purple. ATRA, all-trans retinoic acid; BMP, bone morphogenic protein; EGF, epidermal growth factor; FGF, fibroblast growth factor; OCT4, octamer-binding transcription factor 4; RA, retinoic acid; SAG, smoothened agonist; SCF, stem cell factor; SOX2, sex-determining region Y box; SSEA4, stage-specific embryonic antigen 4; TGFβ, transforming growth factor beta; VEGF, vascular endothelial growth factor. (Color version of figure is available online.)