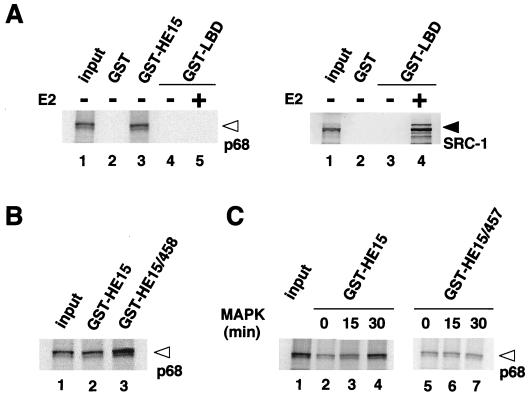
FIG. 4.
The recombinant p68 RNA helicase specifically binds to the hERα A/B domain in vitro. (A) p68 interacts only with the hERα A/B domain but not with the LBD, irrespective of the presence of E2. In vitro-translated p68 RNA helicase recombinant protein (left panel, lane 1) was analyzed by SDS-PAGE (5 to 20% polyacrylamide gradient gel). GST-ER fusion proteins, immobilized on beads, were mixed with 15 μl of in vitro translation reaction mixtures of p68; 2 μl of translation reaction mixture was loaded on the input lane. SRC-1 interacts with the hERα LBD only in the presence of E2 (right panel; lane 4) as previously reported (39). The open arrowhead indicates the position of a p68 RNA helicase protein, and the solid arrowhead indicates the position of the SRC-1 protein. (B) The p68 interaction is enhanced by replacing the Ser118 residue with Glu (S118E) in the bacterially expressed GST fusion protein [GST-HE15/458]. (C) Phosphorylation of the hERα A/B domain by MAPK increases its binding to p68. GST-HE15 or GST-HE15/457 that was incubated with activated MAPK for 0, 15, or 30 min at 30°C was used as the probe for an in vitro pull-down assay with 35S-labeled p68 protein.