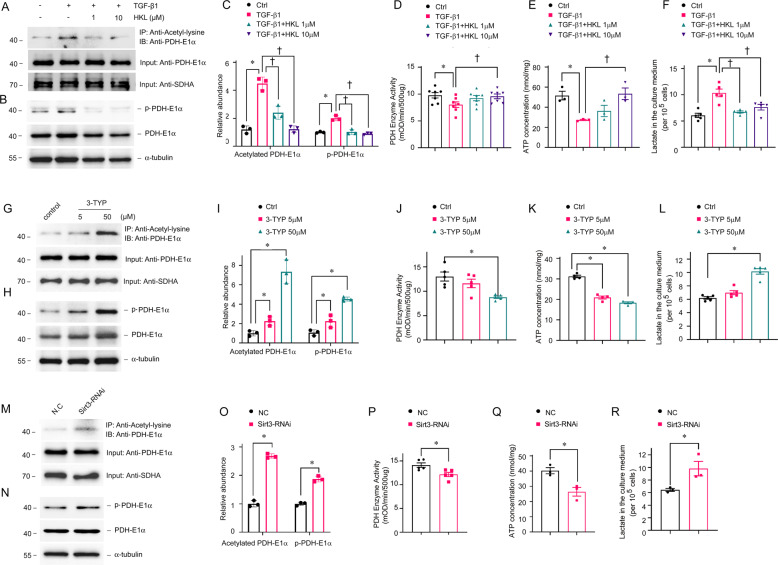
Fig. 7. SIRT3 regulates PDHE1α acetylation and PDH enzyme activity in tubular epithelial cells.
TECs incubated with or without HKL then stimulated with TGF-β1 for 1 h (A–F), TECs stimulated with 3-TYP for 15 min (G–L), and TECs transfected with Sirt3 siRNA (M–R) for 12 h. Mitochondrial lysates immunoprecipitated with anti-acetyl-lysine antibody and analyzed with anti-PDHE1α. (A, C, G, I, M, O). PDHE1α served as the standard (*P < 0.05, †P < 0.05; n = 3). Western blots of phosphorylated and total PDHE1α expression in TEC lysates (B, C, H, I, N, O). PDHE1α served as the standard (*P < 0.05, †P < 0.05; n = 3). Enzyme activity of PDH in TECs incubated with or without HKL, then stimulated with TGF-β1 (*P < 0.05 vs. control, n = 7; †P < 0.05 vs. TGF-β1, n = 7) (D), or 3-TYP (*P < 0.05 vs. vehicle, n = 5) (J), or transfected with Sirt3 siRNA (*P < 0.05 vs. NC siRNA transfection, n = 5) (P); levels of ATP in TECs incubated with or without HKL, then stimulated with TGF-β1 (*P < 0.05 vs. control, n = 3; †P < 0 .05 vs. TGF-β1 stimulation, n = 3) (E), or 3-TYP (*P < 0.05 vs. vehicle, n = 4) (K), or transfected with Sirt3 siRNA (*P < 0.05 vs. NC siRNA transfection, n = 3) (Q); lactate levels in TECs incubated with or without HKL, then stimulated with TGF-β1 (*P < 0.05 compared with control, n = 3; †P < 0.05 compared with TGF-β1 stimulation, n = 3) (F), or by 3-TYP stimulation (*P < 0.05 compared with vehicle treatment, n = 3) (L), or by Sirt3 siRNA transfection (*P < 0.05 compared with NC siRNA transfection, n = 3) (R).