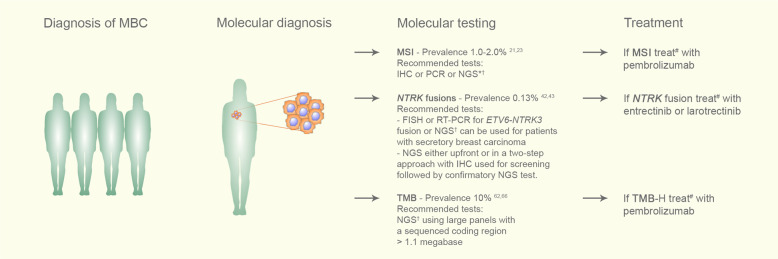
Fig. 2. Suggested flowchart for testing and using approved tumor-agnostic therapies in patients with metastatic breast cancer.
Following the diagnosis of metastatic breast cancer, formalin-fixed, paraffin-embedded (FFPE) tissue blocks from archival tissue or from a new tumor biopsy should be used to evaluate the status of one of the three discussed biomarkers: MSI, TMB-H, or NTRK fusions. In the case of TMB, blood samples can be collected to perform liquid biopsy-based NGS panels. For those patients harboring any of these three biomarkers who have progressed following prior treatment and who have no satisfactory alternative treatment options, we recommend the use of the appropriate tissue-agnostic approved therapy. IHC: immunohistochemistry; FFPE: formalin-fixed, paraffin-embedded; FISH: fluorescence in situ hybridization; MBC: metastatic breast cancer; NGS: next-generation sequencing; PCR: polymerase chain reaction. *In cases of negative IHC for dMMR in breast cancer, confirmatory PCR or NGS could be performed. †NGS should be performed preferentially at validated laboratories. #Treatment with the designated agnostic therapy should be started during the course of therapy for patients with the target biomarker who have progressed following prior treatment, and no satisfactory alternative treatment options are available.