Abstract
Background
It remains unknown whether coronavirus disease 2019 (COVID-19) patients with bipolar disorders (BDs) are at an increased risk of mortality. We aimed to establish whether health outcomes and care differed between patients infected with COVID-19 with BD and patients without a diagnosis of severe mental illness.
Methods
We conducted a population-based cohort study of all patients with identified COVID-19 and respiratory symptoms who were hospitalized in France between February and June 2020. The outcomes were in-hospital mortality and intensive care unit (ICU) admission. We used propensity score matching to control for confounding factors.
Results
In total, 50 407 patients were included, of whom 480 were patients with BD. Patients with BD were 2 years older, more frequently women and had more comorbidities than controls without a diagnosis of severe mental illness. Patients with BD had an increased in-hospital mortality rate (26.6% v. 21.9%; p = 0.034) and similar ICU admission rate (27.9% v. 28.4%, p = 0.799), as confirmed by propensity analysis [odds ratio, 95% confidence interval (OR, 95% CI) for mortality: 1.30 (1.16–1.45), p < 0.0001]. Significant interactions between BD and age and between BD and social deprivation were found, highlighting that the most important inequalities in mortality were observed in the youngest [OR, 95% CI 2.28 (1.18–4.41), p = 0.0015] and most deprived patients with BD [OR, 95% CI 1.60 (1.33–1.92), p < 0.001].
Conclusions
COVID-19 patients with BD were at an increased risk of mortality, which was exacerbated in the youngest and most deprived patients with BD. Patients with BD should thus be targeted as a high-risk population for severe forms of COVID-19, requiring enhanced preventive and disease management strategies.
Key words: Bipolar disorders, COVID-19, health disparities, health services research, psychiatry, public health, real-life data
Introduction
Over 100 million people have been infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and over 1 million have died from coronavirus disease 2019 (COVID-19) worldwide (COVID-19 Map, 2021). Several nationwide studies have suggested that individuals with severe mental disorders may have worse clinical outcomes from COVID-19 (Egede et al., 2021; Fond et al., 2020a, 2020b; Lee et al., 2020; Li, Li, Fortunati, & Krystal, 2020; Nemani et al., 2021; Poblador-Plou et al., 2020; Tzur Bitan et al., 2021; Wang, Xu, & Volkow, 2020). The US and Korean studies reported a global increased risk of COVID-19 mortality in patients with various mental disorders [e.g. bipolar disorders (BDs), schizophrenia, depression, attention deficit hyperactivity disorder, eating disorders, and personality disorders] (Lee et al., 2020; Wang et al., 2020). The French study targeted COVID-19 patients with schizophrenia and reported that increased mortality was not homogeneous according to age or other clinical characteristics, suggesting that COVID-19 health care strategies should be personalized according to vulnerability levels (Fond et al., 2020a, 2020b). A better understanding of the vulnerability to severe COVID-19 in mental disorders other than schizophrenia is an urgent research priority for proposing personalized interventions for COVID-19. To date, no study has targeted COVID-19 patients with BDs, even though BD is one of the most frequent and severe mental disorders, affecting more than 1% of the world's population (McIntyre et al., 2020). BD is associated with a 10–20-year loss of life expectancy, mostly due to cardiovascular diseases and multiple metabolic and addiction comorbidities aggravated by poor compliance with medical follow-up (McIntyre et al., 2020). BD is also associated with socioeconomic deprivation (Schoeyen et al., 2011). All these factors were reported to be associated with an increased risk of severe COVID-19 (Myers, Parodi, Escobar, & Liu, 2020; Schoeyen et al., 2011; Yancy, 2020; Zhou et al., 2020), suggesting that patients with BD may be at an increased risk of COVID-19-related death. In contrast with this hypothesis, a recent large US study suggested that diagnoses of mood disorders were not associated with increased mortality (Nemani et al., 2021). It remains unknown whether COVID-19 patients with BD are at an increased risk of mortality and should be targeted as a high-risk population for severe forms of COVID-19. More information is thus needed about the clinical characteristics, care and outcomes of COVID-19 patients with BD.
In this nationwide study, we aimed to determine whether clinical characteristics, outcomes, and care differed between patients with BD and patients without a diagnosis of severe mental illness. The primary objective was to compare in-hospital mortality between patients with BD and patients without a diagnosis of severe mental illness after adjustment for the main clinical risk factors for COVID-19. The secondary objective was to compare intensive care unit (ICU) admissions between patients with BD and patients without a diagnosis of severe mental illness.
Methods
Study design and data sources
In this population-based cohort study, we used data from the Programme de Médicalisation des Systèmes d'Information (PMSI database), the French national hospital database in which administrative and medical data are systematically collected for acute and psychiatric care. The PMSI database is based on diagnosis-related groups, with all diagnoses coded according to the 10th revision of the International Classification of Diseases (ICD-10) and procedural codes from the Classification Commune des Actes Médicaux. In our study, we included all hospitalized patients in acute care settings between 1 February 2020 and 9 June 2020, aged 15 years or older, with identified COVID-19 (ICD-10 codes: U07.10 or U07.12 or U07.14) and respiratory symptoms (ICD-10 = U07.10 or U07.11), and a length of hospital stay >24 h (to exclude COVID-19 forms that did not require hospitalization), except if the patients died within 24 h. The threshold of 15 years was chosen because it is the usual threshold to distinguish adult psychiatry from pedopsychiatry in France. We excluded patients with a severe mental illness diagnosis other than BD, such as schizophrenia or recurrent major depression (ICD-10 codes = F20*, F22*, F25*, or F33*).
The PMSI database is used to determine financial resources and is frequently and thoroughly verified by both its producer and the paying party, with possible financial and legal consequences for inaccuracies (Boudemaghe & Belhadj, 2017). Data from the PMSI database are deidentified and can be reused for research purposes (Fond et al., 2019b; Revon-Rivière et al., 2019). Due to its suitable accuracy and exhaustive data collection, no patients were lost to follow-up during the study period.
Ethical considerations
Because this study was strictly observational and based on anonymous data, written informed consent from the participants or the authorization from an ethical committee for dealing with human issues was not required in accordance with the French laws.
Procedures
We defined four groups following two steps.
Step 1. Cases were patients who had a diagnosis of BD according to specific ICD-10 codes [i.e. F30* (manic episode) or F31* (bipolar affective disorders)] in either the acute care or psychiatric PMSI database. Controls were patients who did not have a diagnosis of severe mental illness according to specific ICD-10 codes in the acute care PMSI database and who were not listed in the PMSI psychiatry database.
Step 2. To obtain comparability between the groups defined in step 1, we conducted propensity score (PS) matching. Two matched cohorts of patients were then formed: matched cases and matched controls.
Outcomes and collected data
The primary outcome was in-hospital mortality during the index stay for COVID-19. The secondary outcome was ICU admission. We gathered patient sociodemographic data [age classes: <55, 55–65, 65–80 and >80 years; sex: male, female; social deprivation: socially favored, socially deprived (Rey, Jougla, Fouillet, & Hémon, 2009)], clinical data at baseline using ICD-10 codes [smoking addiction: yes, no; weight: normal weight, overweight and obesity; Charlson comorbidity index score categorized as follows: 0, 1–2, 3 and more (Bannay et al., 2016) and main comorbidities: yes, no], stay data (origin of patients: from home or from hospital institution; length of ICU and hospital stay in days), and hospital data (hospital category: public, university and private; number of hospital stays for COVID-19). Social deprivation involves four socioeconomic ecological variables: the proportion of residents who graduated from high school, median household income, the percentage of residents who are blue-collar workers, and the unemployment rate (Rey et al., 2009). It has been categorized into two categories favored v. deprived using zero as the threshold.
Statistical analysis
The baseline characteristics of our study subjects are represented as the counts (proportions) and medians (interquartile ranges) for categorical and continuous variables, respectively.
Analyses were then carried out in two steps. First (step 1), we used either the χ2 test or Student's t test to compare sociodemographic, clinical, and hospital data between cases and unmatched controls. Second (step 2), PSs (Austin, 2011; Marrie, Dawson, & Garland, 2009) were used for matching, considering the following characteristics (p values <0.2 from step 1): age classes (<55, 55–65, 65–75, and ⩾80 years), sex (male, female), social deprivation (favored, deprived), smoking addiction (yes, no), weight (normal weight, overweight, and obesity), and origin of patients (from home or from hospital institution). In addition, all Charlson comorbidities (yes, no) were included regardless of the p-value. We matched patients with the closest PS inside the hospital to control for confounders at the hospital level using a greedy 1:20 max algorithm without replacement and requiring that the logit of the PS of a patient with BD and one without a diagnosis of severe mental illness be within 0.20 standard deviations of one another. Age, sex, and dementia were exactly matched due to persistent heterogeneity between groups, even when these variables were involved in the PS score. Standardized differences were used to assess the balance between the matched groups for baseline characteristics using weights to normalize the distribution of patients with BD to equal the number of patients without a diagnosis of severe mental illness. An absolute standardized difference (SD) of ⩽0.20 was chosen to indicate a negligible difference in the mean or prevalence of a variable between groups. To study the association between BD and the two outcomes, the odds ratio (OR) and 95% confidence interval (95% CI) were estimated using a multivariable generalized linear model (logit function), with a robust variance estimator to account for clustering within matched pairs. Several models were performed for each outcome: model 1 without adjustment; model 2: adding to model 1 the variables with the most important differences after PS matching (SD > 0.1); model 3 (only for mortality): adding ICU admission to model 1; and model 4: adding to model 2 three interaction terms (BD × age, BD × sex, and BD × social deprivation) to check whether the association between BD and the two outcomes was homogenous according to age, sex, and social deprivation. This interaction was determined based on a previous study reporting the influence of age on the COVID-19 prognosis in schizophrenia (Fond et al., 2020a, 2020b; Lakbar, Luque-Paz, Mege, Einav, & Leone, 2020; Lewis et al., 2020).
To test the robustness of our results, three series of sensitivity analyses were performed. We repeated the analyses for the two outcomes: (i) on the two subgroups of patients: F30* (manic episode) or F31* (bipolar affective disorders); (ii) by completing the matching approach described above with exact matching for tobacco smoking and a caliper = 0.10; and (iii) by matching on all the variables with SD > 0.05 using a narrower caliper = 0.05.
A significance threshold of p < 0.05 was used. All analyses were performed using SAS (version 9.4).
Results
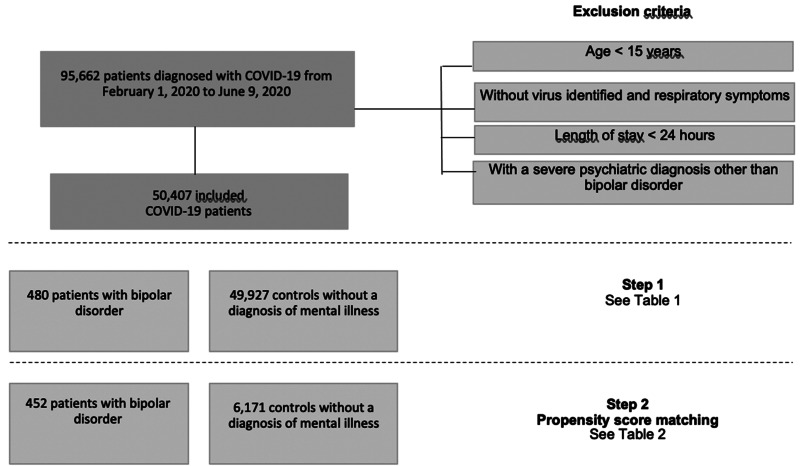
Overall, 95 662 patients with COVID-19 were hospitalized between 1 February 2020 and 9 June 2020. After excluding patients aged <15 years, those without identified virus and respiratory symptoms, those with a length of stay <24 h (except if they died at the hospital) and those with a diagnosis of schizophrenia or recurrent major depressive disorder, 50 407 patients were included in the current study (flow chart, Fig. 1). The overall in-hospital mortality rate was 21.8%.
Fig. 1.
Flow chart.
In the first step, 480 patients with BD (449 with F31 code and 31 with F30 code) were compared to 49 927 controls without a diagnosis of severe mental illness (Table 1). Compared to controls, patients with BD had an increased in-hospital mortality rate (26.0% v. 21.7%, p = 0.0341), with no difference in the ICU admission rate (27.9% v. 28.4%, p = 0.7985). In addition, patients with BD were slightly older [mean 70.92 standard deviation (13.48) v. 68.72 (17.07), p < 0.0001], more frequently women (62.1% v. 43.1%, p < 0.0001), more socially favored (57.9% v. 48.6%, p = 0.0037), particularly in patients with BD older than 55 years, more frequently diagnosed with smoking addiction (9.0% v. 4.2%, p = 0.0003), higher Charlson comorbidity score (p = 0.0002), more frequent chronic renal disease (16.5% v. 12.1%, p = 0.0106), dementia (21.3% v. 9.6%, p < 0.0001), cerebrovascular disease (9.8% v. 6.3%, p = 0.0084), and chronic obstructive pulmonary disease (18.5% v. 12.6%, p = 0.0021). They were less frequently admitted from home (84.8% v. 90.3%, p = 0.0020) and had a shorter length of hospital stay [median 9-day interquartile range (6–19) v. 12 days (5–16)]. The distribution of hospitalizations by month was similar between patients with BD and controls (online Supplementary Fig. S1).
Table 1.
Baseline characteristics and outcomes of hospitalized COVID-19 patients with BD and without a diagnosis of severe mental illness before PS matching
| Characteristics | Total n = 50 407 n (%) |
Patients with BD n = 480 n (%) |
Controls n = 49 927 n (%) |
p-value |
|---|---|---|---|---|
| Sociodemographic data | ||||
| Age (years) | <0.0001 | |||
| <55 | 10 458 (20.8) | 50 (10.4) | 10 408 (20.9) | |
| 55–65 | 8536 (16.9) | 88 (18.3) | 8448 (16.9) | |
| 65–80 | 15 377 (30.5) | 200 (41.7) | 15 177 (30.4) | |
| ⩾80 | 16 036 (31.8) | 142 (29.6) | 15 894 (31.8) | |
| Sex | <0.0001 | |||
| Male | 28 598 (56.7) | 182 (37.9) | 28 416 (56.9) | |
| Female | 21 809 (43.3) | 298 (62.1) | 21 511 (43.1) | |
| Social deprivation index | 0.0037 | |||
| More favored | 24 517 (48.6) | 278 (57.9) | 24 239 (48.6) | |
| More deprived | 23 961 (47.5) | 186 (38.8) | 23 775 (47.6) | |
| Missing | 1929 (3,8) | 16 (3.3) | 1913 (3.8) | |
| Clinical data at baseline | ||||
| Smoking addiction | 2135 (4.2) | 43 (9.0) | 2092 (4.2) | 0.0003 |
| Weighta | 0.0792 | |||
| Normal weight | 43 259 (85.8) | 395 (82.3) | 42 864 (85.9) | |
| Overweight | 1168 (2.3) | 12 (2.5) | 1156 (2.3) | |
| Obesity | 5.907 (11.8) | 73 (15.2) | 5.980 (11.9) | |
| Comorbidities | ||||
| Charlson comorbidity index score | 0.0002 | |||
| 0 | 20 724 (41.1) | 146 (30.4) | 20 578 (41.2) | |
| 1–2 | 17 364 (34.5) | 187 (39.0) | 17 177 (34.4) | |
| ⩾3 | 12 319 (24.4) | 147 (30.6) | 12 172 (24.4) | |
| Renal disease | 6102 (12.1) | 79 (16.5) | 6023 (12.1) | 0.0106 |
| Peripheral vascular disease | 2865 (5.7) | 27 (5.6) | 2838 (5.7) | 0.9972 |
| Hemiplegia or paraplegia | 2139 (4.2) | 28 (5.8) | 2111 (4.2) | 0.1029 |
| Cancer | 4473 (8.9) | 42 (8.8) | 4431 (8.9) | 0.8925 |
| HIV or AIDS | 303 (0.6) | 4 (0.8) | 299 (0.6) | 0.5834 |
| Diabetes with complications | 3093 (7.3) | 35 (7.3) | 3058 (6.1) | 0.3067 |
| Diabetes without complications | 10 958 (23.5) | 113 (23.5) | 10 845 (21.7) | 0.3224 |
| Dementia | 4878 (21.3) | 102 (21.3) | 4776 (9.6) | <0.0001 |
| Cerebrovascular disease | 3175 (6.3) | 47 (9.8) | 3128 (6.3) | 0.0084 |
| Chronic obstructive pulmonary disease | 6392 (12.7) | 89 (18.5) | 6303 (12.6) | 0.0021 |
| Congestive heart failure | 8141 (16.2) | 73 (15.2) | 8068 (16.2) | 0.6260 |
| Myocardial infarction | 3729 (7.4) | 27 (5.6) | 3702 (7.4) | 0.1791 |
| Stay data | ||||
| Origin of the patient | 0.0020 | |||
| From home | 45 510 (90.3) | 407 (84.8) | 45 103 (90.3) | |
| Hospital data | ||||
| Hospital category | 0.0554 | |||
| Public | 29 648 (58.8) | 259 (54.0) | 29 389 (58.9) | |
| University | 16 711 (33.2) | 187 (39.0) | 16 524 (33.1) | |
| Private | 4048 (8.0) | 34 (7.1) | 4014 (8.0) | |
| Outcomes | ||||
| In hospital mortality | 10.979 (21.8) | 125 (26.0) | 10.854 (21.7) | 0.0341 |
| ICU admission | 14.290 (28.4) | 134 (27.9) | 14.156 (28.4) | 0.7985 |
N: effective; %: percentage; IQR: interquartile range; BD: bipolar disorder.
p-value in bold: statistical significance.
Body mass index: <25: normal weight; 25–30: overweight; >30: obesity.
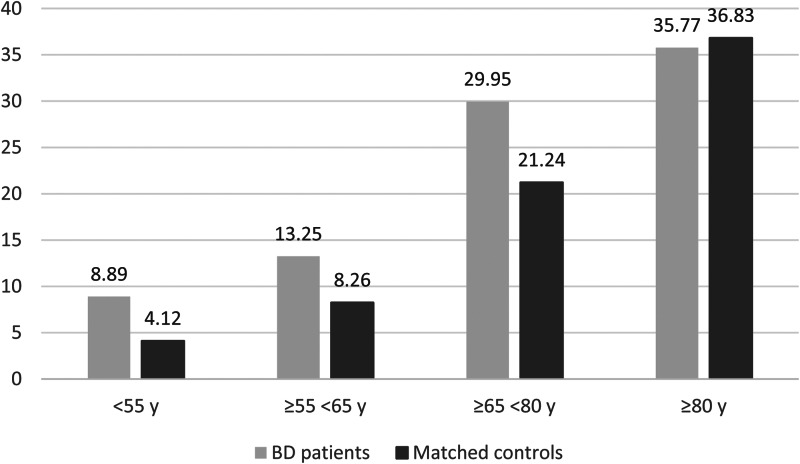
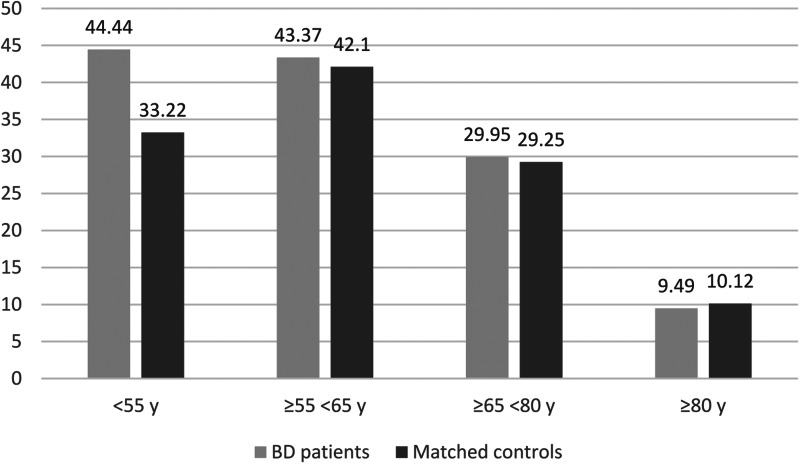
In the second step, 452 patients with BD were matched with 6171 controls without a diagnosis of severe mental illness (Table 2). The patients with BD were found to have an increased in-hospital mortality rate compared to their matched controls (26.6% v. 21.4%) regardless of the tested models, while no significant difference was observed for ICU admission (27.7% v. 26.2%) (Figs 2 and 3; Table 3). Significant interactions between BD and age and between BD and social deprivation for in-hospital mortality were found, highlighting that the most important inequalities in mortality were observed in the youngest (OR, 95% CI 2.28 (1.18–4.41), p = 0.0015) and most deprived patients with BD (OR, 95% CI 1.60 (1.33–1.92), p < 0.001). The youngest patients with BD had more direct ICU admission with a more severe state of health than controls. They were also more likely to require invasive mechanical ventilation (online Supplementary File 1).
Table 2.
Baseline characteristics and outcomes of hospitalized COVID-19 patients with BD and without a diagnosis of severe mental illness after PS matching
| Characteristics | Patients with BD n = 452 n (weighted%) |
Controls n = 6171 n (weighted%) |
SD |
|---|---|---|---|
| Sociodemographic data | |||
| Age as continuous (mean ± standard deviation) | 71.1 ± 13.6 | 71.6 ± 4.0 | −0.05 |
| Age (years) | |||
| <55 | 45 (10.0) | 721 (10.0) | 0.00 |
| 55–65 | 83 (18.4) | 1193 (18.4) | 0.00 |
| 65–80 | 187 (41.4) | 2468 (41.4) | 0.00 |
| ⩾80 | 137 (30.3) | 1789 (30.3) | 0.00 |
| Sex | |||
| Male | 176 (38.9) | 2512 (38.9) | 0.00 |
| Female | 276 (61.1) | 3659 (61.1) | 0.00 |
| Social deprivation index | |||
| More favored | 267 (59.1) | 3586 (56.2) | 0.06 |
| More deprived | 170 (37.6) | 2366 (40.7) | −0.06 |
| Missing | 15 (3.3) | 219 (3.1) | 0.01 |
| Clinical data at baseline | |||
| Smoking addiction | 39 (8.6) | 272 (3.9) | 0.17 |
| Weighta | 0.09 | ||
| Normal weight | 373 (82.5) | 5221 (86.2) | −0.10 |
| Overweight | 12 (2.7) | 175 (2.4) | 0.01 |
| Obesity | 67 (14.8) | 775 (11.3) | 0.10 |
| Comorbidities | |||
| Charlson comorbidity index score | |||
| 0 | 143 (31.6) | 2400 (35.9) | −0.09 |
| 1–2 | 173 (38.3) | 2198 (37.7) | 0.01 |
| ⩾3 | 136 (30.1) | 1573 (26.4) | 0.08 |
| Renal disease | 75 (16.6) | 760 (12.3) | 0.12 |
| Peripheral vascular disease | 26 (5.8) | 339 (6.1) | −0.01 |
| Hemiplegia or paraplegia | 24 (5.3) | 274 (6.7) | 0.03 |
| Cancer | 39 (8.6) | 605 (9.5) | −0.03 |
| HIV or AIDS | 4 (0.9) | 42 (0.8) | 0.01 |
| Diabetes with complications | 33 (7.3) | 383 (6.8) | 0.02 |
| Diabetes without complications | 106 (23.5) | 1375 (22.6) | 0.02 |
| Dementia | 86 (19.0) | 775 (19.0) | 0.00 |
| Cerebrovascular disease | 41 (9.1) | 408 (6.8) | 0.08 |
| Chronic obstructive pulmonary disease | 81 (17.9) | 797 (130.) | 0.14 |
| Congestive heart failure | 71 (15.7) | 1016 (16.7) | −0.02 |
| Myocardial infarct | 27 (6.0) | 432 (7.5) | −0.06 |
| Stay data | |||
| Origin of the patient | −0.06 | ||
| From home | 384 (85.0) | 5466 (87.2) | |
| Hospital data | |||
| Hospital category | 0.00 | ||
| Public | 237 (52.4) | 2826 (52.4) | |
| University | 187 (41.4) | 3100 (41.4) | |
| Private | 28 (6.2) | 245 (6.2) | |
| Outcomes | |||
| In hospital mortality | 120 (26.6%) | 1394 (21.9%) | 0.11 |
| ICU admission | 125 (27.7%) | 1686 (26.2%) | 0.03 |
N, effective; %, percentage; IQR, interquartile range; BD, bipolar disorder; SD, standardized difference.
p-Value in bold: statistical significance.
SD > 0.2 indicated an important imbalance, and SD > 0.1 was included in the multivariate models.
Body mass index: <25: normal weight; 25–30: overweight; >30: obesity.
Fig. 2.
In-hospital mortality by age classes after propensity score matching.
Fig. 3.
ICU admission by age classes after propensity score matching.
Table 3.
Compared outcomes between the two matched groups
| In-hospital mortality | ICU admission | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Model 1 | 1.30 (1.16–1.45) | <0.0001 | 1.08 (0.97–1.20) | 0.1778 |
| Model 2 | 1.28 (1.14–1.44) | <0.0001 | 1.02 (0.91–1.14) | 0.7558 |
| Model 3 | 1.29 (1.15–1.44) | <0.0001 | – | – |
| Model 4 | ||||
| Interaction term age × bipolar disorder | <0.0001 | 0.0536 | ||
| <55 years | 2.28 (1.18–4.41) | 0.0015 | 1.63 (1.19–2.25) | 0.0027 |
| 55–65 years | 1.70 (1.17–2.47) | 0.0050 | 1.06 (0.84–1.33) | 0.6485 |
| 65–80 years | 1.59 (1.34–1.90) | <0.0001 | 1.04 (0.88–1.22) | 0.6865 |
| ⩾80 years | 0.95 (0.80–1.15) | 0.6164 | 0.93 (0.69–1.25) | 0.6325 |
| Interaction term Sex × bipolar disorder |
0.8589 | 0.0306 | ||
| Male | 1.31 (1.10–1.55) | 0.0021 | 0.94 (0.80–1.11) | 0.4769 |
| Female | 1.28 (1.10–1.49) | 0.0013 | 1.20 (1.04–1.39) | 0.0147 |
| Interaction term social deprivation × bipolar disorder | 0.0013 | 0.9996 | ||
| Deprived level | 1.60 (1.33–1.92) | <0.0001 | 1.08 (0.90–1.28) | 0.4149 |
| Favored level | 1.17 (1.00–1.35) | 0.0434 | 1.08 (0.93–1.24) | 0.3054 |
ICU, intensive care unit.
Model 1: unadjusted model.
Model 2: model 1 plus adjustment for variables with SD > 0.1.
Model 3: model 1 plus adjustment for ICU admission.
Model 4: model 2 plus the interaction terms. Reference: matched controls.
OR (95% CI): odds ratio (95% confidence interval).
p-Value in bold: statistical significance.
In the sensitivity analyses, the results were maintained when excluding the 31 patients with F30* code (manic episode) [adjusted OR for mortality: 1.30 (1.16–1.45), p < 0.0001, p > 0.05 for ICU admission, analyses not shown], with the exact matching for tobacco smoking and caliper = 0.10 [adjusted OR for mortality: 1.30 (1.13–1.45), p < 0.0001, p > 0.05 for ICU admission, see online Supplementary File 2] and with the matching on all the variables with SD > 0.05 using caliper = 0.05 [adjusted OR for mortality: 1.27 (1.04–1.54), p = 0.017, p > 0.05 for ICU admission, analyses not shown].
Discussion
To our knowledge, this is the largest series of patients with BD with COVID-19 reported to date, including 480 patients with BD hospitalized for COVID-19 infection with a nationwide geographical distribution. COVID-19 patients with BD were at an increased risk of mortality after adjustment for clinical risk factors for COVID-19, which was exacerbated in the youngest and most deprived patients with BD. In contrast to patients with schizophrenia, no difference was reported for ICU admission (Fond et al., 2020a, 2020b).
Our prematching findings inform us about BD patient characteristics, highlighting several risk factors for COVID-19 aggravation (i.e. obesity, renal disease, dementia and cerebrovascular disease, and chronic obstructive pulmonary disease). Obesity has been reported as a risk factor for COVID-19 hospital admission and worse outcomes (Lighter et al., 2020). In a nationwide study, hospitalized COVID-19 patients with chronic renal disease had significantly higher mortality than those without renal disease (Ozturk et al., 2020). A preexisting diagnosis of dementia was found to be an independent risk factor for COVID-19 hospitalization and mortality (Atkins et al., 2020). Chronic obstructive pulmonary disease has also been reported to worsen the progression and prognosis of COVID-19 (Zhao et al., 2020). Beyond the strict question of COVID-19, these data are a strong reminder of the need to pursue efforts to prevent and manage somatic diseases in BD (Heiberg et al., 2019; Nielsen, Kugathasan, Straszek, Jensen, & Licht, 2019). In contrast, one BD patient characteristic was less expected. Patients with BD were largely more socially favored than controls, although BD is known to induce impaired professional and social functioning and poor socioeconomic outcomes (Hakulinen, Elovainio, et al., 2019; Hakulinen, Musliner, & Agerbo, 2019). Social deprivation is an important cause of premature mortality among patients with BD (Fond et al., 2019a; Martin et al., 2014), which can thus explain a broader representation of socially favored patients with BD, particularly in the oldest patients with BD. The overrepresentation of socially favored patients with BD may also suggest lower access to hospital care in deprived patients with BD than in controls. Previous studies have suggested that, among deprived individuals, patients with severe mental illnesses, such as BD, are some of the most underserved and undertreated populations (Nielsen et al., 2019; Tinland et al., 2020). In a recent study on homeless individuals, the main negative predictors for hospital use were a lower social functioning score and BDs (Loubière et al., 2020). Further studies are warranted to explore disease management during the period between the onset of symptoms and hospitalization in patients with BD.
In contrast with a recent US study reporting that mood disorders were not associated with increased mortality (Nemani et al., 2021), we found that COVID-19 patients with BD were at an increased risk of mortality after adjustment for the main clinical risk factors for COVID-19 (i.e. age, obesity, renal disease, dementia and cerebrovascular disease, and chronic obstructive pulmonary disease). This discrepancy suggests that mortality is not homogeneous within affective disorders and that patients with BD should be targeted as a high-risk population for severe forms of COVID-19. Several factors can be advanced to explain this health inequity between BD and the general population. Abnormal mood may alter decision-making or treatment adherence, which may impact prognosis. A delay in obtaining medical attention and in access to hospital care may explain the higher severity of patients with BD, as suggested in the youngest patients with BD who had more frequent direct ICU admissions. The barriers to access to somatic care for patients with BD have been described in previous studies (Hernández-Gómez, Andrade-González, Lahera, & Vieta, 2020). Immunological disturbances have also been suggested to play an important role in the mortality of young patients with BD without classic comorbidities (Lu et al., 2021). Human leukocyte antigen predominantly regulates viral infection, especially COVID-19 (Tamouza, Krishnamoorthy, & Leboyer, 2021), and has recently been suggested to play a role in the etiology of BD (Tamouza et al., 2018). Patients with BD may also be at higher risk of hypovitaminosis D, contributing to poor COVID-19 prognosis (Di Nicola et al., 2020; Hastie et al., 2020). Psychotropic drugs are also modulators of the immune system. Lithium, widely used in treating BD, also exhibits antiviral activity that has not been shown to protect patients with BD (Ishii, Terao, & Hirakawa, 2021). Antipsychotics are used in both schizophrenia and BD treatment and modify immune dysfunction (May, Slitzky, Rostama, Barlow, & Houseknecht, 2020), which may explain the shared increased mortality between BD and schizophrenia patients (26% in both populations) (Fond et al., 2020a, 2020b). The background regimen of patients with BD may also have drug interactions with anti-COVID-19 therapies, such as antimicrobials or immunomodulators. Patients with BD are often treated with long-term benzodiazepines. They may therefore require higher doses of sedatives due to tolerance issues, which may lead to increased complications. Thus, in the ICU, these patients may require muscle relaxant agents more frequently, which may lead to increased complications upon mechanical ventilation withdrawal. Further studies are warranted to better understand and to address modifiable factors that can prevent this increased mortality in COVID-19 patients with BD. Our study also underscores the exacerbation of health disparities in the youngest and most deprived patients with BD. Targeted interventions are needed for the youngest and most deprived patients with BD to reduce these stark disparities.
Strengths
The current study is the first national-based study of COVID-19 mortality in patients with BD. Many confounding factors were captured in the PS matching, which ensured the robustness of our results. The PMSI has a system of coding at discharge with strict variable definitions (primary and secondary diagnoses using the ICD-10; as well as procedure codes associated with the care provided) and a subset of records audited on a regular basis to avoid excessively high rates of coding errors.
Limits
Only the in-hospital mortality rate was explored in the current study. Ambulatory variables (treatment observance, medical follow-up, and social isolation) and ethnicity were not captured in the database and have therefore not been explored and may have impacted the COVID-19 prognosis. Some data are known to be insufficiently coded in medico-administrative databases (e.g. tobacco smoking and obesity) (Fond et al., 2020a, 2020b) and lifestyle factors were not captured. The mood state of the patients was not available in the PMSI database, and it is not known whether an acute manic or depressed episode may have impacted the COVID-19 prognosis of patients with BD. As the precise cause of death was not captured in the database, we cannot definitely conclude that COVID-19 was the cause of death. However, the patients died in median during the 11 days following hospital admission for COVID-19 infection, we can therefore reasonably assume that COVID-19 infection played an important role in their death. As mentioned in our rationale, our purpose was to explore specifically BDs, the most severe adult psychiatric disorder with schizophrenia that was already explored in our previous paper (Fond et al., 2020a, 2020b). These diagnoses are the most well identified and coded psychiatric diagnoses in the PMSI database, contrary to other mood or personality disorders, especially if patients have not been hospitalized or are followed-up in the private sector. Future studies should specifically explore mood and personality disorders considering these limitations.
Conclusion
COVID-19 patients with BD were at an increased risk of mortality after adjustment for the main clinical risk factors for COVID-19 (i.e. age, obesity, renal disease, dementia and cerebrovascular disease, and chronic obstructive pulmonary disease), suggesting the existence of other factors that lead to this health inequity (i.e. delay in access to care, immunological disturbances, and the effects of psychotropic drugs). This increased mortality was exacerbated in the youngest and most deprived patients with BD. COVID-19 patients with BD should thus be targeted as a high-risk population for severe forms of COVID-19, requiring enhanced preventive care and disease management strategies.
Acknowledgements
The authors thank David Braunstein, Myriam Dubuc, Cyprien Fabre, Marie-Thérèse Jimeno, Vincent Pradel, Anne Remacle, Fanny Romain, Catherine Seyler, Françoise Volot, and all the other members of the Department of Medical Information.
Author contributions
Veronica Orleans and Vanessa Pauly had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Laurent Boyer and Guillaume Fond. Acquisition, analysis, or interpretation of data: Pascal Auquier, Karine Baumstarck, Laurent Boyer, Christophe Lancon, Pierre-Michel Llorca, Guillaume Fond, Marc Leone, Vanessa Pauly, and Veronica Orleans. Drafting of the manuscript: Laurent Boyer and Guillaume Fond. Critical revision of the manuscript for important intellectual content: All the authors. Statistical analysis: Vanessa Pauly. Administrative, technical, or material support: Veronica Orleans. Supervision: Laurent Boyer.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291721003676.
click here to view supplementary material
Conflict of interest
The authors declare no competing interests.
References
- Atkins, J. L., Masoli, J. A. H., Delgado, J., Pilling, L. C., Kuo, C.-L., Kuchel, G. A., & Melzer, D. (2020). Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(11), 2224–2230. 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, P. C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research, 46(3), 399–424. 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannay, A., Chaignot, C., Blotière, P.-O., Basson, M., Weill, A., Ricordeau, P., & Alla, F. (2016). The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Medical Care, 54(2), 188–194. 10.1097/MLR.0000000000000471. [DOI] [PubMed] [Google Scholar]
- Boudemaghe, T., & Belhadj, I. (2017). Data resource profile: The French national uniform hospital discharge data set database (PMSI). International Journal of Epidemiology, 46(2), 392–392d. 10.1093/ije/dyw359. [DOI] [PubMed] [Google Scholar]
- COVID-19 Map. (2021). Johns Hopkins Coronavirus Resource Center. Available at https://coronavirus.jhu.edu/map.html. January 28.
- Di Nicola, M., Dattoli, L., Moccia, L., Pepe, M., Janiri, D., Fiorillo, A., … Sani, G. (2020). Serum 25-hydroxyvitamin D levels and psychological distress symptoms in patients with affective disorders during the COVID-19 pandemic. Psychoneuroendocrinology, 122, 104869. 10.1016/j.psyneuen.2020.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egede, J., Campbell, J. A., Walker, R. J., Garacci, E., Dawson, A. Z., & Egede, L. E. (2021). Relationship between physical and mental health comorbidities and COVID-19 positivity, hospitalization, and mortality. Journal of Affective Disorders, 283, 94–100. 10.1016/j.jad.2021.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond, G., Pauly, V., Bege, T., Orleans, V., Braunstein, D., Leone, M., … Boyer, L. (2019a). Trauma-related mortality of patients with severe psychiatric disorders: Population-based study from the French national hospital database. The British Journal of Psychiatry: The Journal of Mental Science, 1–7. 10.1192/bjp.2019.139. [DOI] [PubMed] [Google Scholar]
- Fond, G., Salas, S., Pauly, V., Baumstarck, K., Bernard, C., Orleans, V., … Boyer, L. (2019b). End-of-life care among patients with schizophrenia and cancer: A population-based cohort study from the French national hospital database. The Lancet. Public Health, 4(11), e583–e591. 10.1016/S2468-2667(19)30187-2. [DOI] [PubMed] [Google Scholar]
- Fond, G., Pauly, V., Leone, M., Llorca, P.-M., Orleans, V., Loundou, A., … Boyer, L. (2020a). Disparities in intensive care unit admission and mortality Among patients with schizophrenia and COVID-19: A national cohort study. Schizophrenia Bulletin, 47(3), 624–634. 10.1093/schbul/sbaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond, G., Pauly, V., Orleans, V., Antonini, F., Fabre, C., Sanz, M., … Boyer, L. (2020b). Increased in-hospital mortality from COVID-19 in patients with schizophrenia. L'Encephale, 47(2), 89–95. 10.1016/j.encep.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen, C., Elovainio, M., Arffman, M., Lumme, S., Pirkola, S., Keskimäki, I., … Böckerman, P. (2019). Mental disorders and long-term labour market outcomes: Nationwide cohort study of 2 055 720 individuals. Acta Psychiatrica Scandinavica, 140(4), 371–381. 10.1111/acps.13067. [DOI] [PubMed] [Google Scholar]
- Hakulinen, C., Musliner, K. L., & Agerbo, E. (2019). Bipolar disorder and depression in early adulthood and long-term employment, income, and educational attainment: A nationwide cohort study of 2390127 individuals. Depression and Anxiety, 36(11), 1080–1088. 10.1002/da.22956. [DOI] [PubMed] [Google Scholar]
- Hastie, C. E., Mackay, D. F., Ho, F., Celis-Morales, C. A., Katikireddi, S. V., Niedzwiedz, C. L., … Pell, J. P. (2020). Vitamin D concentrations and COVID-19 infection in UK biobank. Diabetes & Metabolic Syndrome, 14(4), 561–565. 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiberg, I. H., Jacobsen, B. K., Balteskard, L., Bramness, J. G., Naess, Ø., Ystrom, E., … Høye, A. (2019). Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatrica Scandinavica, 139(6), 558–571. 10.1111/acps.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Gómez, A., Andrade-González, N., Lahera, G., & Vieta, E. (2020). Recommendations for the care of patients with bipolar disorder during the COVID-19 pandemic. Journal of Affective Disorders, 279, 117–121. 10.1016/j.jad.2020.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, N., Terao, T., & Hirakawa, H. (2021). Association between trace levels of lithium in drinking water and COVID-19-associated mortality. Bipolar Disorders, 23(1), 100–100. 10.1111/bdi.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakbar, I., Luque-Paz, D., Mege, J.-L., Einav, S., & Leone, M. (2020). COVID-19 gender susceptibility and outcomes: A systematic review. PLoS One, 15(11), e0241827. 10.1371/journal.pone.0241827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. W., Yang, J. M., Moon, S. Y., Yoo, I. K., Ha, E. K., Kim, S. Y., … Yon, D. K. (2020). Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: A nationwide cohort study. The Lancet Psychiatry, 7(12), 1025–1031. 10.1016/S2215-0366(20)30421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, N. M., Friedrichs, M., Wagstaff, S., Sage, K., LaCross, N., Bui, D., … Dunn, A. (2020). Disparities in COVID-19 incidence, hospitalizations, and testing, by area-level deprivation – Utah, March 3–July 9, 2020. MMWR. Morbidity and Mortality Weekly Report, 69(38), 1369–1373. 10.15585/mmwr.mm6938a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., Li, F., Fortunati, F., & Krystal, J. H. (2020). Association of a prior psychiatric diagnosis with mortality among hospitalized patients with coronavirus disease 2019 (COVID-19) infection. JAMA Network Open, 3(9), e2023282. 10.1001/jamanetworkopen.2020.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighter, J., Phillips, M., Hochman, S., Sterling, S., Johnson, D., Francois, F., … Stachel, A. (2020). Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clinical Infectious Diseases, 71(15), 896–897. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubière, S., Tinland, A., Taylor, O., Loundou, A., Girard, V., Boyer, L., & Auquier, P. (2020). Determinants of healthcare use by homeless people with schizophrenia or bipolar disorder: Results from the French housing first study. Public Health, 185, 224–231. 10.1016/j.puhe.2020.05.019. [DOI] [PubMed] [Google Scholar]
- Lu, Y., Huang, Z., Wang, M., Tang, K., Wang, S., Gao, P., … Zhao, J. (2021). Clinical characteristics and predictors of mortality in young adults with severe COVID-19: A retrospective observational study. Annals of Clinical Microbiology and Antimicrobials, 20(1), 3. 10.1186/s12941-020-00412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie, R. A., Dawson, N. V., & Garland, A. (2009). Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. Journal of Clinical Epidemiology, 62(5), 511–517.e1. 10.1016/j.jclinepi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Martin, J. L., McLean, G., Park, J., Martin, D. J., Connolly, M., Mercer, S. W., & Smith, D. J. (2014). Impact of socioeconomic deprivation on rate and cause of death in severe mental illness. BMC Psychiatry, 14(1), 261. 10.1186/s12888-014-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, M., Slitzky, M., Rostama, B., Barlow, D., & Houseknecht, K. L. (2020). Antipsychotic-induced immune dysfunction: A consideration for COVID-19 risk. Brain, Behavior, & Immunity – Health, 6, 100097. 10.1016/j.bbih.2020.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, R. S., Berk, M., Brietzke, E., Goldstein, B. I., López-Jaramillo, C., Kessing, L. V., … Mansur, R. B. (2020). Bipolar disorders. Lancet (London, England), 396(10265), 1841–1856. 10.1016/S0140-6736(20)31544-0. [DOI] [PubMed] [Google Scholar]
- Myers, L. C., Parodi, S. M., Escobar, G. J., & Liu, V. X. (2020). Characteristics of hospitalized adults With COVID-19 in an integrated health care system in California. JAMA, 323(21), 2195–2198. 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani, K., Li, C., Olfson, M., Blessing, E. M., Razavian, N., Chen, J., … Goff, D. C. (2021). Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry, 78(4), 380–386. 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, R. E., Kugathasan, P., Straszek, S., Jensen, S. E., & Licht, R. W. (2019). Why are somatic diseases in bipolar disorder insufficiently treated? International Journal of Bipolar Disorders, 7(1), 12. 10.1186/s40345-019-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk, S., Turgutalp, K., Arici, M., Odabas, A. R., Altiparmak, M. R., Aydin, Z., … Ates, K. (2020). Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: A nationwide analysis from Turkey. Nephrology, Dialysis, Transplantation, 35(12), 2083–2095. 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblador-Plou, B., Carmona-Pírez, J., Ioakeim-Skoufa, I., Poncel-Falcó, A., Bliek-Bueno, K., Cano-Del Pozo, M., … EpiChron Group, null. (2020). Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: Results from the PRECOVID study in Spain. Int. J.Environ. Res. Public Health, 17(14), 5171. 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revon-Rivière, G., Pauly, V., Baumstarck, K., Bernard, C., André, N., Gentet, J.-C., … Boyer, L. (2019). High-intensity end-of-life care among children, adolescents, and young adults with cancer who die in the hospital: A population-based study from the French national hospital database. Cancer, 125(13), 2300–2308. 10.1002/cncr.32035. [DOI] [PubMed] [Google Scholar]
- Rey, G., Jougla, E., Fouillet, A., & Hémon, D. (2009). Ecological association between a deprivation index and mortality in France over the period 1997–2001: Variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health, 9(1), 33. 10.1186/1471-2458-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeyen, H. K., Birkenaes, A. B., Vaaler, A. E., Auestad, B. H., Malt, U. F., Andreassen, O. A., & Morken, G. (2011). Bipolar disorder patients have similar levels of education but lower socio-economic status than the general population. Journal of Affective Disorders, 129(1–3), 68–74. 10.1016/j.jad.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Tamouza, Krishnamoorthy, R., & Leboyer, M. (2021). Understanding the genetic contribution of the human leukocyte antigen system to common major psychiatric disorders in a world pandemic context. Brain, Behavior, and Immunity, 91, 731–739. 10.1016/j.bbi.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamouza, Oliveira, J., Etain, B., Bengoufa, D., Hamdani, N., Manier, C., … Leboyer, M. (2018). HLA genetics in bipolar disorder. Acta Psychiatrica Scandinavica, 138(5), 464–471. 10.1111/acps.12912. [DOI] [PubMed] [Google Scholar]
- Tinland, A., Loubière, S., Boucekine, M., Boyer, L., Fond, G., Girard, V., & Auquier, P. (2020). Effectiveness of a housing support team intervention with a recovery-oriented approach on hospital and emergency department use by homeless people with severe mental illness: A randomised controlled trial. Epidemiology and Psychiatric Sciences, 29, e169. 10.1017/S2045796020000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur Bitan, D., Krieger, I., Kridin, K., Komantscher, D., Scheinman, Y., Weinstein, O., … Feingold, D. (2021). COVID-19 prevalence and mortality among schizophrenia patients: A large-scale retrospective cohort study. Schizophrenia Bulletin, 47(5), 1211–1217. 10.1093/schbul/sbab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., Xu, R., & Volkow, N. D. (2020). Increased risk of COVID-19 infection and mortality in people with mental disorders: Analysis from electronic health records in the United States. World Psychiatry, 20(1), 124–130. 10.1002/wps.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy, C. W. (2020). COVID-19 and African Americans. JAMA, 323(19), 1891–1892. 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- Zhao, Q., Meng, M., Kumar, R., Wu, Y., Huang, J., Lian, N., … Lin, S. (2020). The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. Journal of Medical Virology, 1915–1921. 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., … Cao, B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet (London, England), 395(10229), 1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291721003676.
click here to view supplementary material