Abstract
Background
Acute acoustic trauma (AAT) is an acute hearing impairment caused by intense noise-impact. The current management strategy for AAT with substantial hearing loss in the Dutch military is the combination therapy with corticosteroids and hyperbaric oxygen therapy (HBOT). In a previous study, early initiation of the combination therapy was associated with better outcomes. Therefore, we performed a new analysis to assess the difference in hearing outcome between patients in whom combination therapy was started within two days, versus after more than two days.
Methods
A retrospective analysis was performed on military patients diagnosed with AAT with substantial hearing loss who presented between February 2018 and March 2020. Absolute and relative hearing improvement between first and last audiograms were calculated for all affected frequencies (defined as loss of ≥20 dB on initial audiogram). We also determined the amount of patients who recovered to the level of Dutch military requirement, and performed speech discrimination tests.
Results
In this analysis, 30 male patients (49 ears) with AAT were included. The median age was 24.5 years (IQR 23–29). The median time to initiation of therapy with corticosteroids and HBOT were one and two days, respectively. HBOT was started within two days in 31 ears, and after more than two days in 18 ears. The mean absolute and relative hearing gains were 18.8 dB (SD 14.6) and 46.8% (SD 31.3) on all affected frequencies. The 100% discrimination/speech perception level improved from 64.0 dB to 51.7 dB (gain 12.3 dB ± 14.1). There was significantly more improvement in absolute and relative hearing improvement when HBOT was started in ≤2 days, compared to >2 days.
Conclusion
Our analysis shows results in favor of early initiation (≤2 days) of the combination treatment of HBOT and corticosteroids in patients with AAT.
Keywords: Hyperbaric oxygen therapy, Acute acoustic trauma, Hearing loss, Corticosteroids, Blast injury, Noise-induced hearing loss
1. Background
Acute acoustic trauma (AAT) is an acute hearing impairment caused by intense noise-impact. AAT is relatively common in the military due to exposure to high intensity sounds in shooting and blasts (Bayoumy et al., 2020). Although novel and emerging treatments for hearing loss are being developed (Schilder et al., 2019; Crowson et al., 2017), according to a Cochrane review (Wei et al., 2013) no specific drug therapy has been proven effective in ameliorating hearing loss in AAT. The current management strategy for AAT in the Dutch military is corticosteroids, or in case of substantial hearing loss, the combination treatment of hyperbaric oxygen therapy (HBOT) with corticosteroids (Bayoumy and de Ru, 2019). In a previous study, we showed that the combination of HBOT and corticosteroids was significantly better in terms of hearing improvement compared to corticosteroid monotherapy (Bayoumy et al., 2020). Another finding was that earlier start of therapy was associated with higher relative hearing gains. To further investigate this latter finding, we performed a new analysis on patients who entered the database after our first study to assess the effect of combination therapy with corticosteroids and HBOT, with special interest for early (≤2 days) versus later (>2 days) initiation of HBOT.
2. Methods
We retrospectively reviewed all patients diagnosed with AAT and considered for HBOT between January 2018 and March 2020 at the Department of Otolaryngology, Central Military Hospital Utrecht, the Netherlands. Exclusion was based on the amount of hearing loss on the first audiogram (tested frequencies 250, 500, 1000, 2000, 3000, 4000, 6000, and 8000 Hz). If hearing loss was less than 30 dB on at least one, 25 dB on at least two, or 20 dB on three or more frequencies as compared with the contralateral ear or with a recent (military service entry) audiogram, then patients were not included in this analysis. All included patients were offered combination therapy. Patients were not analyzed for the current analysis if they were not treated with HBOT (i.e. they chose monotherapy with corticosteroids), if there were missing audiograms or if they were lost to follow up. The patients underwent standard otolaryngological examination and were treated with prednisolone 60 mg for 7 days and 10 daily sessions of HBOT. HBOT was performed in a multi-person recompression chamber, where patients breathe 100% oxygen at a pressure of 243 kPA, in four periods of 20 min oxygen separated by three “air breaks” of 5 min duration. The total treatment time is 110 min including compression and decompression.
2.1. Hearing outcomes
The hearing outcomes were calculated as absolute (Eq. (1)) and relative (Eq. (2)) hearing improvements using the contralateral ear (or the recent audiogram) as baseline as described by Plontke et al. (2007) Outcomes were determined based on the latest available audiogram. The majority of follow-up audiograms were taken at least 4 weeks after treatment. The included frequencies in this analysis were 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz. In each individual patient, a frequency was deemed ‘affected’ if the difference was 20 dB or more compared with the unaffected contralateral ear, or a recent previous audiogram of the ipsilateral ear.
In case of bilateral involvement and for assessment of functional impairment we used the Dutch military hearing standard, which requires a maximum hearing impairment of 20 dB at frequencies ≤2000 Hz and 30 dB at frequencies ≥3000 Hz.
The equations are:
| (1) |
| (2) |
where pre indicates before therapy, post indicates after therapy and contralateral indicates the contralateral ear. The mean absolute and relative hearing improvement at affected frequencies was calculated for all patients (Eq. (1)). If the contralateral ear was also affected, the ‘contralateral ear’ was set at 0 dB using equation (2), for calculation's sake.
Speech recognition was also determined in this analysis. The speech audiogram was determined using the Netherlands Society of Audiology wordlist. The standard curve includes the percentage adequately spoken phonemes as function of the sounds volume in dB. We assessed the lowest intensity at which 100% discrimination was obtained.
In addition, we also determined if patients recovered to the hearing level required for Dutch military service (defined above). If these criteria were not met, the patient would theoretically be considered ‘unsuitable’ for military service and thus ‘not recovered’ from the AAT.
Since our analysis objective was to determine the effect of early versus later initiation of therapy, we created two subgroups of patients: HBOTearly with the patients in whom HBOT was started within two days after AAT, and HBOTlate with the patients in whom HBOT was started more than two days after AAT.
2.2. Statistical analysis
IBM® SPSS® Statistics Version 24 IBM (IBM Corp., Armonk, New York, U.S.) was used for statistical analysis. The Shapiro-Wilk test was used to test for normality. Data were expressed as mean ± SD, n (%) or median ± IQR. If normally distributed, the χ2 test or Student's t-test was used for group comparisons. We accepted a two-sided p-value less than 0.05 as statistically significant. This analysis is reported according to the STROBE statement (von Elm et al., 2008).
3. Results
3.1. Baseline characteristics
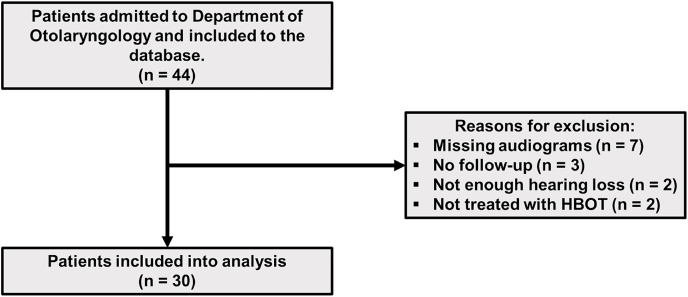
Between January 2018 and March 2020, 44 patients with AAT were considered for HBOT at our department (Fig. 1). Fourteen patients were excluded from this analysis due to missing audiograms, lack of follow-up, insufficient hearing loss, or the fact that they did not receive HBOT. Thirty patients (49 ears) with AAT were included in the final analysis. The median age of patients was 24.5 years (IQR 23–29), all patients were male and none had any comorbidities. No Eustachian tube dysfunction was found. There were no significant differences in demographics between both HBOTearly and HBOTlate groups. The amount of past noise exposure was not documented in the patients’ records. The acoustic trauma was caused by gunfire in all patients. This could have been with all the different weaponry used by our armed forces. The exact intensity and amount of exposed shots were not documented. The median time to corticosteroids and HBOT were one (IQR 1–2) and two days (IQR 2–4). The median time to HBOT in the HBOTearly and HBOTlate groups were two (IQR 1.75–2) and four (IQR 3–5.25) days. The median duration of follow-up was 1.0 month (IQR 1–3). In total, 30 patients (49 ears) were treated with the combination therapy of HBOT and corticosteroids.
Fig. 1.
Flowchart of included patients in this analysis.
3.2. Hearing outcomes
The mean hearing level at initial and last audiograms on the combined affected frequencies were 38.2 dB ± 12.2 and 19.4 dB ± 12.7. The absolute and relative hearing gains on these frequencies were 18.8 dB ± 14.6 and 46.8% ± 31.3. The outcomes on the individual affected frequencies can be found in Table 1. The mean hearing level at which the affected ear could discriminate 100% of words correctly (speech recognition test) at initial and last audiograms was 64.0 dB ± 13.5 and 51.7 dB ± 6.6, an increase of 12.3 dB ± 14.1 (n = 45 ears, P = 0.0001, paired T-test). A subgroup analysis was performed between patients treated within two days (HBOTearly, (n = 31 ears) compared with those who were treated after more than two days (HBOTlate, (n = 18 ears). The initial hearing loss was 38.7 dB ± 12.9 and 37.2 dB ± 11.3 in the HBOTearly and HBOTlate groups respectively, P = 0.66. The absolute hearing gain was 22.9 dB ± 14.1 in the HBOTearly group, whereas the HBOTlate group had an absolute hearing gain of 11.6 dB ± 12.9 (P = 0.007). The relative hearing gains were 56.3% ± 28.0 and 30.6% ± 30.6, for the HBOTearly and HBOTlate groups, respectively (P = 0.004). Seventeen (57%) out of 30 AAT patients recovered to the hearing level required for Dutch military service. In the HBOTearly group, 10 (63%) out of 16 patients returned to adequate hearing levels, while seven (50%) out of 14 patients in the HBOTlate group returned to adequate hearing levels (P = 0.49, Chi-Square-test).
Table 1.
Outcomes of early treatment of HBOT and corticosteroids combination therapy. Note: in none of the patients hearing was affected at 250, 500, or 1000 Hz and therefore these frequencies are not reported.
| Initial audiogram (dB) | Last audiogram (dB) | Absolute hearing gain (dB) | Relative hearing gain (%) | |
|---|---|---|---|---|
| Audiometry | ||||
| 2000 Hz (n = 16) | 31.9 ± 11.9 | 11.3 ± 8.7 | 20.6 ± 16.8 | 57.3 ± 38.4 |
| 3000 Hz (n = 28) | 42.7 ± 15.2 | 18.2 ± 12.9 | 24.5 ± 18.5 | 52.3 ± 35.3 |
| 4000 Hz (n = 30) | 44.7 ± 16.5 | 23.3 ± 16.5 | 21.3 ± 17.2 | 46.8 ± 33.3 |
| 6000 Hz (n = 40) | 41.6 ± 16.8 | 22.9 ± 16.6 | 18.8 ± 16.0 | 44.4 ± 34.1 |
| 8000 Hz (n = 39) | 40.6 ± 16.4 | 20.6 ± 14.3 | 20.0 ± 16.5 | 46.6 ± 30.6 |
| All frequencies (n = 49) | 38.2 ± 12.2 | 19.4 ± 12.7 | 18.8 ± 14.6 | 46.8 ± 31.3 |
| Speech recognition test (n = 45) | 64.0 dB ± 13.5 at 100% | 51.7 dB ± 6.6 at 100% | 12.3 dB ± 14.1 | – |
| Timing of HBOT initiation | ||||
| ≤ two days (n = 31) | 38.7 ± 12.9 | 15.9 ± 9.8 | 22.9 ± 14.1 | 56.3 ± 28.0 |
| > two days (n = 18) | 37.2 ± 11.3 | 24.3 ± 15.6 | 11.6 ± 12.9 | 30.6 ± 30.6 |
| P-value | 0.66 | 0.02 | 0.007 | 0.004 |
4. Discussion
In this retrospective analysis, the effectiveness of the combination treatment of HBOT and corticosteroids for AAT was assessed. Our analysis shows a good overall hearing recovery following combination therapy. Furthermore, we suggest that HBOT initiation within two days after onset of symptoms was associated with better outcomes than HBOT initiation after more than two days.
4.1. Early initiation of HBOT
The timing of HBOT is controversial, because in preclinical studies very early initiation of HBOT after AAT led to worsened hearing outcomes. D'Aldin et al. (d'Aldin et al., 1999) reported that HBOT monotherapy worsened AAT-induced cochlear thresholds by approximately 10–15 dB, after it was initiated 1h after AAT. However the combination therapy of HBOT and corticosteroids did result in improvement of threshold shifts. Very early initiation of HBOT monotherapy within 1 h after AAT showed lower hearing gains compared to the control group in an animal study by Kahraman et al. (2012) However, they did find significant hearing improvements in the group that was treated with HBOT and corticosteroids. Cakir et al. (2006) reported worsening hearing thresholds when HBOT was administered 1h after AAT, while it showed protective effects when administered 2, 6 and 24 h after AAT. There was some recovery in the HBOT group that was treated 48h after trauma, however the difference was not significant compared to the control group (no treatment).
Fakhry (Fakhry et al., 2007), Arslan (Arslan et al., 2012) and Ylikoski (Ylikoski et al., 2008) reported benefits of HBOT after it was administered at least 24h post AAT. Fakhry et al. (2007) reported significant reductions in threshold shifts in animals who were exposed to noise (115 dB at 8 kHz) who received the combination group of HBOT and corticosteroids compared to the therapies individually. Significantly improved threshold shifts were found at 8 kHz (+8.6 dB), 10 kHz (+13.4 dB) and 12.5 kHz (+10 dB) in the group of patients who received combination therapy within one day. In the group of patients who received the combination therapy after 6 days, still a significant difference was found in threshold shifts on 8 kHz (+7.7 dB) and 10 kHz (+8.5 dB).
Arslan et al. (2012) found that in rats that were treated with HBOT monotherapy within 3h after AAT, higher significant higher levels of pro-inflammatory IL-1β were found compared to the control group. When HBOT monotherapy was started within 24 h no significant difference was found in the level of pro-inflammatory parameters compared to the control group. Furthermore, post-treatment auditory brain stem responses were better compared to control and HBOT 3h groups. Dexamethasone monotherapy also showed a similar pro-inflammatory profile compared to the HBOT 24h and control groups, and a significant improvement of post-treatment auditory brain stem responses.
Furthermore, Ylikoski et al. (2008) compared HBOT monotherapy versus normobaric oxygen therapy (NBOT) monotherapy for the treatment of AAT. The treatments in this study were initiated within 48h. The average PTA (0.5, 1 and 2 kHz) losses were 13.2 (SD 9.2) and 13.7 (SD 9.2) for the HBOT and NBOT groups. The corresponding relative hearing gains for the HBOT and NBOT groups were 74.1% (SD 19.9) and 60.2% (SD 28.9), P < 0.001).
The average HPTA (4, 6 and 8 kHz) losses were 37.1 (SD 14.4) and 37.3 (SD 15.2) for the HBOT and NBOT groups. The relative hearing gains for both HBOT and NBOT groups were 69.3% (SD 17.1) and 56.2% (SD 20.3), P < 0.001).
4.2. Clinical outcomes from literature: early HBOT and corticosteroids
Lafère et al. (2010) compared three groups of treatment in a Belgian military population: group 1 treated with only oral methylprednisolone and oral piracetam, group 2 treated with a combination of HBOT (once daily for 10 days, started ≥ 36h after AAT), oral methylprednisolone and oral piracetam, and group 3 treated with HBOT (twice daily for three days, followed by once daily for 7 seven days, started < 36h after AAT) in combination with methylprednisolone and piracetam both administered intravenously. The mean initial hearing losses (250–8000 Hz) in the three groups were 25.8 dB ± 11.7 (group 1), 31.4 dB ± 19.0 (group 2) and 29.7 dB ± 15.7 (group 3). The mean absolute hearing gains were 5.6 dB (≈57%) ± 3.58 (group 1), 20.6 (≈66%) dB ± 17.7 (group 2) and 17.0 (≈57%) dB ± 14.0 (group 3). Both HBOT groups showed significantly better recovery compared to the non-HBOT group. Bayoumy et al. (2020) compared HBOT and corticosteroids combination therapy with corticosteroids monotherapy for AAT in the Dutch military. In that study, 29 patients were included in the combination group and 24 patients in the corticosteroid group. The initial hearing losses were 46.1 dB ± 14.4 and 38.6 dB ± 11.3 for the combination and corticosteroids only groups, respectively. The mean absolute hearing gains were 23.5 dB ± 12.1 and 12.5 dB ± 12.5 for the combination and corticosteroids monotherapy groups, respectively. This difference was statistically significant. Furthermore, patients who were treated early (≤2 days) with HBOT and corticosteroids had a mean relative hearing gain of 71.4% ± 27.5, while patients treated > 2 days after trauma had a relative hearing gain of 47.9% ± 31.6 (p < 0.05). In the present analysis, we obtained a relative hearing gain of 56.3% ± 28.0 for patients treated within two days with the combination therapy, while those treated later had a relative hearing gain of 30.6% ± 30.6. It seems that the results from our previous report were slightly better than the current results. This may be due to higher mean initial losses in the previous report. Another possibility is that in the current analysis there might have been pre-existing hearing loss in some of the patients. Unfortunately, no recent previous audiogram from before the acoustic trauma were available. From both our studies and that of Lafère et al. (2010), it can be summarized that the relative hearing gains from HBOT patients treated within two days ranged between 56.3 and 71.4%, while the relative hearing gains for patients not receiving HBOT were ranging between 21.7 and 29.9%. This strongly suggest that there is a necessity to treat patients as early as possible. Furthermore, Salihoğlu et al. (2015) also reported better results in patients treated with HBOT and corticosteroids within 10 days after trauma (group A) compared to patients treated after 11–30 days after trauma (group B). The mean time between AAT and initiation of HBOT was 7.4 days (SD 2.0) and 18.9 days (SD 7.0) for group A and B, respectively. They found that eight patients (22%) had complete or partial hearing recovery in group A, while group B had only three (8%) patients with partial recovery. Ahmed et al. (2021) recently published a systematic review and meta-analysis on the efficacy of HBOT and/or corticosteroids in AAT. In the meta-analysis (random effects model, I2 = 62%), they found that the mean PTA (0.5, 1 and 2 kHz) difference was 7.0 dB (CI-95% 0.8–13.2 dB) between pre- and post-treatment thresholds in studies that investigated the combination therapy. Furthermore, in their meta-analysis they found (random effects model, I2 = 69%) of HPTA (4, 6 and 8 kHz) pre- and post-treatment hearing thresholds that the mean difference was 12.4 dB (CI-95% 4.0–20.9). This suggests an effect of HBOT and corticosteroids for the treatment of AAT. Table 2 summarizes all studies that we could identify that investigated the effect of HBOT in AAT.
Table 2.
Summary of all studies on HBOT for AAT(Bayoumy and de Ru, 2019; van der Veen et al., 2014). PF: pentoxifylline, Dx: dextran, VD: vasodilators, OS: oral steroids, IVS: intravenous steroids, PT: piracetam, NBOT: normobaric oxygen therapy, HBOT: hyperbaric oxygen therapy, IV: intravenous, MT: medical therapy, NA: not available.
| Author, year | Groups that included HBOT | Groups that did not include HBOT | Absolute hearing gain (dB) | Relative hearing gain (%) |
|---|---|---|---|---|
| Current analysis, 2021 | HBOT + OS | None | 24.5 dB | 58.4% |
| Bayoumy (Bayoumy et al., 2020), 2019 | HBOT + OS | OS | HBOT + OS: 23.5 dB OS: 12.5 dB |
HBOT + OS: 57.6% OS: 31.4% |
| Oya (Oya et al., 2019), 2019 | 1: HBOT + OS 2: HBOT - OS |
None | NA | HBOT + OS: 42.5% HBOT - OS: 25.4% |
| Van Haesendonck (Van Haesendonck et al., 2018), 2018 | HBOT + OS | OS | HBOT: 2.5 dB OS: 3.0 dB |
NA |
| Salihoğlu (Salihoğlu et al., 2015), 2015 | 1: HBOT + OS ≤ 10d 2: HBOT + OS > 10d |
None | NA | NA |
| Bonfort (Bonfort et al., 2014), 2014 | HBOT + IVS + PF | None | 18.3 dB | 53.8% |
| Lafère (Lafère et al., 2010), 2010 | 1: HBOT (o.d.for 10 days) + OS + PT 2: HBOT (b.d. for three days, followed by o.d. for 7 days) + IVS + PT IV |
MT: OS + PT | 1: 17.0 dB 2: 20.6 dB MT: 5.6 dB |
NA |
| Ylikoski (Ylikoski et al., 2008), 2008 | HBOT | NBOT | NA | PTA (0.5, 1, 2 kHz) HBOT: 74.1% NBOT: 60.2% P < 0.001 PTA (4, 6, 8 kHz) HBOT: 69.3% NBOT: 56.2% P < 0.001 |
| Winiarski (Winiarski et al., 2005), 2005 | HBOT + PT | None | NA | NA |
| Vavrina (Vavrina and Müller, 1995), 1995 | HBOT + OS + Dx | OS + Dx | NA | NA |
| Pilgramm (Pilgramm and Schumann, 1985), 1985 | HBOT + Sorbitol + Dx + betahistine | None | NA | NA |
| Demaertelare (Demaertelaere and Van Opstal, 1981), 1981 | HBOT | None | NA | NA |
| De Heyn (De Heyn and Van Opstal, 1976), 1976 | HBOT + VD | VD | NA | NA |
4.3. Strength and limitations
The organizational aspects of the medical care for Dutch military patients with AAT provide a strong framework for the present analysis. For instance, The Netherlands has only one military hospital, to which all serious cases of AAT are referred. There are short communication lines between the otolaryngology department and the Royal Navy Diving Medical Center, which ensures early initiation of HBOT. Military AAT patients make up a homogeneous population of otherwise healthy individuals. A limitation of this analysis is that it has a retrospective design that consequently lacks a control group and blinding. Therefore, we cannot exclude the possibility that part of the hearing improvement is due the natural course of the condition (spontaneous recovery) and/or reduction of a temporary threshold shift. Given the retrospective nature of the current evidence of HBOT for AAT. We recommend to perform a randomized controlled trial for early initiation of HBOT and corticosteroids in patients with AAT. It is essential that in such trial important factors such as timing of audiograms and timing of treatment are equally distributed among treatment arms.
5. Conclusion
Our analysis shows improved hearing recovery in terms of absolute and relative hearing gain, in case of early (≤two days) versus late (>two days) initiation of combination therapy with HBOT and corticosteroids following AAT.
Consent for publication
All authors agreed with the final version of the manuscript.
Funding
No funding was obtained for the study.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Ahmed M.M., Allard R.J., Esquivel C.R. Noise-induced hearing loss treatment: systematic review and meta-analysis. Mil. Med. 2021 doi: 10.1093/milmed/usaa579. [DOI] [PubMed] [Google Scholar]
- Arslan H.H., Satar B., Serdar M.A., Ozler M., Yilmaz E. Effects of hyperbaric oxygen and dexamethasone on proinflammatory cytokines of rat cochlea in noise-induced hearing loss. Otol. Neurotol. 2012;33(9):1672–1678. doi: 10.1097/MAO.0b013e31826bf3f6. [DOI] [PubMed] [Google Scholar]
- Bayoumy A.B., de Ru J.A. The use of hyperbaric oxygen therapy in acute hearing loss: a narrative review. Eur. Arch. Oto-Rhino-Laryngol. 2019;276(7):1859–1880. doi: 10.1007/s00405-019-05469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumy A.B., van der Veen E.L., van Ooij P.A.M. Effect of hyperbaric oxygen therapy and corticosteroid therapy in military personnel with acute acoustic trauma. BMJ Mil Health. 2020;166(4):243–248. doi: 10.1136/jramc-2018-001117. [DOI] [PubMed] [Google Scholar]
- Bonfort G., Billot D., Trendel D., Salf E., Lindas P., Barberot J.P. [Acute acoustic trauma, a retrospective analysis about 225 military cases] Rev. Laryngol. Otol. Rhinol. 2014;135(1):25–31. [PubMed] [Google Scholar]
- Cakir B.O., Ercan I., Civelek S. Negative effect of immediate hyperbaric oxygen therapy in acute acoustic trauma. Otol. Neurotol. 2006;27(4):478–483. doi: 10.1097/01.mao.0000224080.77849.3d. [DOI] [PubMed] [Google Scholar]
- Crowson M.G., Hertzano R., Tucci D.L. Emerging therapies for sensorineural hearing loss. Otol. Neurotol. : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017;38(6):792–803. doi: 10.1097/MAO.0000000000001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Aldin C., Cherny L., Devrière F., Dancer A. Treatment of acoustic trauma. Ann. N. Y. Acad. Sci. 1999;884:328–344. doi: 10.1111/j.1749-6632.1999.tb08652.x. [DOI] [PubMed] [Google Scholar]
- De Heyn G., Van Opstal M. [Comparative study of acoustic trauma caused by blasts, treated by vasodilators or by a combination of vasodilators and hyperbaric oxygenation] Acta Oto-Rhino-Laryngol. Belg. 1976;30(3):251–259. [PubMed] [Google Scholar]
- Demaertelaere L., Van Opstal M. [Treatment of acoustic trauma with hyperbaric oxygen] Acta Oto-Rhino-Laryngol. Belg. 1981;35(3–4):303–314. [PubMed] [Google Scholar]
- Fakhry N., Rostain J.C., Cazals Y. Hyperbaric oxygenation with corticoid in experimental acoustic trauma. Hear. Res. 2007;230(1):88–92. doi: 10.1016/j.heares.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Kahraman E., Ata N., Incesulu A., Bal C. The role of different agents in the prevention of the negative effects of immediate hyperbaric oxygen therapy in acute acoustic trauma. Journal of International Advanced Otology. 2012;8:158–165. [Google Scholar]
- Lafère P., Vanhoutte D., Germonprè P. Hyperbaric oxygen therapy for acute noise-induced hearing loss: evaluation of different treatment regimens. Diving Hyperb Med. 2010;40(2):63–67. [PubMed] [Google Scholar]
- Oya M., Tadano Y., Takihata Y., Ikomi F., Tokunaga T. Utility of hyperbaric oxygen therapy for acute acoustic trauma: 20 years' experience at the Japan maritime self-defense force undersea medical center. Int. Arch. Otorhinolaryngol. 2019;23(4):e408–e414. doi: 10.1055/s-0039-1688433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgramm M., Schumann K. Hyperbaric oxygen therapy for acute acoustic trauma. Arch. Oto-Rhino-Laryngol. 1985;241(3):247–257. doi: 10.1007/BF00453696. [DOI] [PubMed] [Google Scholar]
- Plontke S.K., Bauer M., Meisner C. Comparison of pure-tone audiometry analysis in sudden hearing loss studies: lack of agreement for different outcome measures. Otol. Neurotol. 2007;28(6):753–763. doi: 10.1097/mao.0b013e31811515ae. [DOI] [PubMed] [Google Scholar]
- Salihoğlu M., Ay H., Cincik H. Efficiency of hyperbaric oxygen and steroid therapy in treatment of hearing loss following acoustic trauma. Undersea Hyperb. Med. 2015;42(6):539–546. [PubMed] [Google Scholar]
- Schilder A.G.M., Su M.P., Blackshaw H. Hearing protection, restoration, and regeneration: an overview of emerging therapeutics for inner ear and central hearing disorders. Otol. Neurotol. 2019;40(5):559–570. doi: 10.1097/MAO.0000000000002194. [DOI] [PubMed] [Google Scholar]
- van der Veen E.L., van Hulst R.A., de Ru J.A. Hyperbaric oxygen therapy in acute acoustic trauma: a rapid systematic review. Otolaryngol. Head Neck Surg. 2014;151(1):42–45. doi: 10.1177/0194599814526555. [DOI] [PubMed] [Google Scholar]
- Van Haesendonck G., Van Rompaey V., Gilles A., Topsakal V., Van de Heyning P. Otologic outcomes after blast injury: the brussels bombing experience. Otol. Neurotol. 2018;39(10) doi: 10.1097/MAO.0000000000002012. [DOI] [PubMed] [Google Scholar]
- Vavrina J., Müller W. Therapeutic effect of hyperbaric oxygenation in acute acoustic trauma. Rev. Laryngol. Otol. Rhinol. 1995;116(5):377–380. [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Wei B.P.C., Stathopoulos D., O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst. Rev. 2013;(7) doi: 10.1002/14651858.CD003998.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiarski M., Kantor I., Smereka J., Jurkiewicz D. [Effectiveness of pharmacologic therapy combined with hyperbaric oxygen in sensorineural hearing loss following acute acoustic trauma. Preliminary report] Pol. Merkur. Lek. 2005;19(111):348–350. [PubMed] [Google Scholar]
- Ylikoski J., Mrena R., Makitie A., Kuokkanen J., Pirvola U., Savolainen S. Hyperbaric oxygen therapy seems to enhance recovery from acute acoustic trauma. Acta Otolaryngol. 2008;128(10):1110–1115. doi: 10.1080/00016480801901634. [DOI] [PubMed] [Google Scholar]