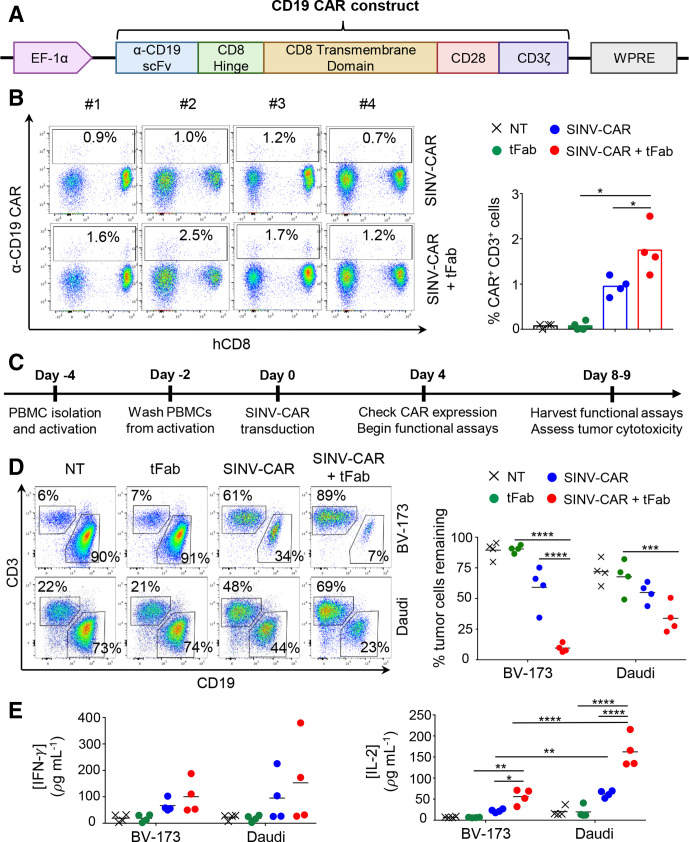
Figure 2.
T cells transduced with SINV-CAR in combination with tFab express functional CD19.CAR and eliminate tumor B cells in vitro. (A) Schematic representation of the CD19.CAR cassette under the control of the EF-1α promoter and WPRE post-transcriptional regulatory molecule. (B) Flow cytometry plots (left) and summary (right) showing CAR expression in T cells transduced with SINV-CAR or SINV-CAR plus tFab. Non-transduced (NT) and tFab alone samples of T cells are provided as negative controls (n=4, mean shown). *P=0.0437 SINV-CAR plus tFab versus SINV-CAR; *p=0.0100 SINV-CAR plus tFab versus tFab with paired t-test. (C) Experimental schema for the transduction and subsequent coculturing of CAR-T cells with tumor B cells in vitro. (D) Representative flow plots (left panel) and summary (right panel) of the quantification of residual CD19+ tumor B cells (BV-173 and Daudi cell lines) remaining after coculturing with either NT, tFab, SINV-CAR, or SINV-CAR plus tFab treated T cells (E:T=2:1). All cells were collected after 4 or 5 days (BV-173 and Daudi, respectively) and stained with CD3 and CD19 mAbs to identify T cells and tumor cells, respectively, by flow cytometry (n=4, mean shown). ***P=0.0004, ****p<0.0001, two-way ANOVA. (E) Quantification of IFN (left panel) and IL-2 (right panel) cytokines in supernatant collected after 24 hours of coculturing NT, tFab, SINV-CAR, or SINV-CAR plus tFab treated T cells with tumor cell lines (E:T=2:1) (n=4, mean shown). *P=0.0393, **p<0.0087, ****p<0.0001, two-way ANOVA. ANOVA, analysis of variance; CAR, chimeric antigen receptor; tFab, tandem Fab format.