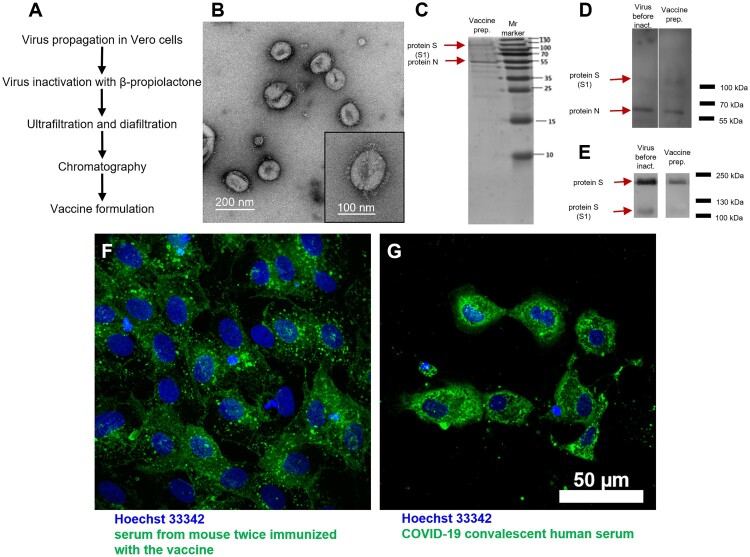
Figure 1.
CoviVac vaccine production process and identity testing: (A) Principal structure of the vaccine production process. (B) TEM of the negatively stained inactivated vaccine concentrate after chromatographic purification; representative particle (bottom right). (C) SDS-PAGE of the vaccine preparation (purified inactivated virus preparation after chromatography), stained with Coomassie blue. (D) Western blot of the virus preparation before inactivation and vaccine preparation (purified inactivated virus preparation after chromatography), stained with COVID-19 convalescent human serum. (E) Western blot of the virus preparation before inactivation and vaccine preparation (purified inactivated virus preparation after chromatography), stained with anti-S protein rabbit serum. (F) Vero cells infected with SARS-CoV-2 and stained with serum of a CoviVac-immunized mouse (green) and Hoechst 33342 (blue). (G) Vero cells infected with SARS-CoV-2 and stained with COVID-19 convalescent human serum (green) and Hoechst 33342 (blue)