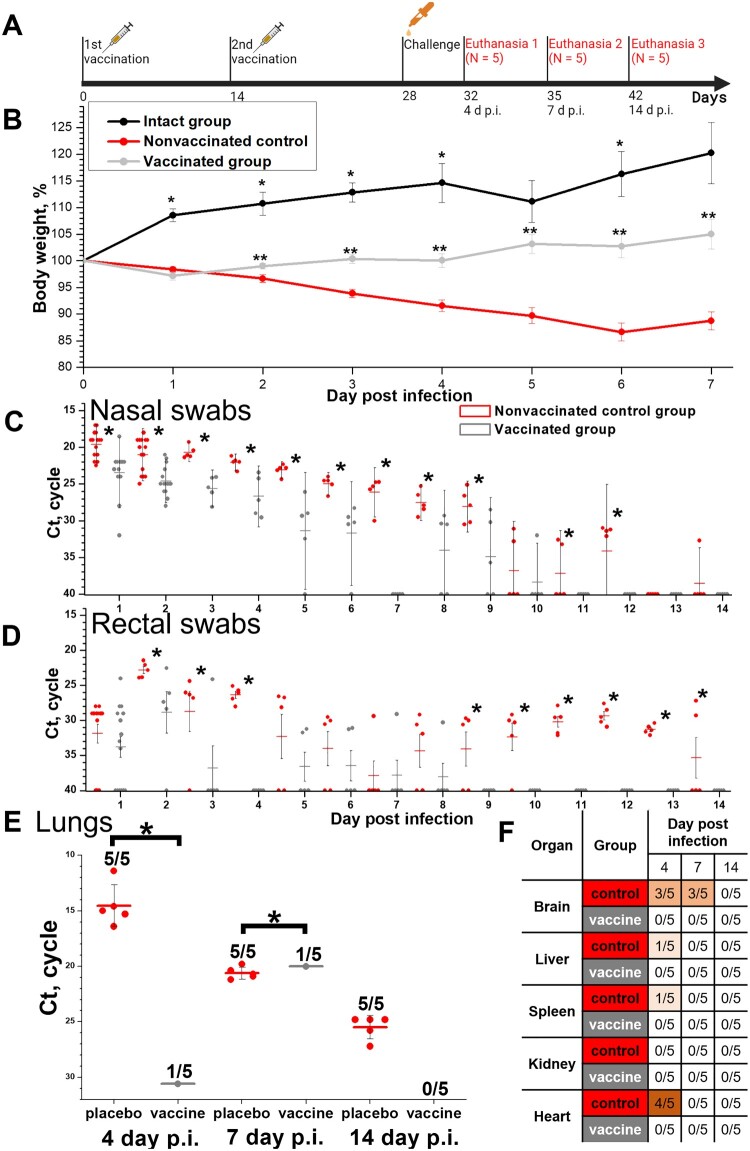
Figure 4.
Protective efficacy in Syrian hamsters (N = 15, 5 animals were euthanized on days 4, 7 and 14 p.i.) of the CoviVac vaccine (6 μg per dose, 2 immunizations with a 14-day interval) against intranasal challenge 14 days after the 2nd immunization with 105 TCID50 of SARS-CoV-2 strain PIK35. (A) Scheme of the experiment (visualized by BioRender). (B) Body weights of intact hamsters and challenged vaccinated and control groups. *Differences between intact and vaccinated groups are statistically significant (Mann–Whitney, p<0.05) **Differences between vaccinated and control groups are statistically significant (Mann–Whitney, p < 0.05). (C) Viral RNA presence in nasal swabs of challenged vaccinated and control hamsters on days 1–14 post infection. Line shows Mean, whiskers show ± SD. *Differences between control and vaccinated groups are statistically significant (Mann–Whitney test, p < 0.05). (D) Viral RNA presence in rectal swabs of challenged vaccinated and control hamsters on days 1–14 post infection. Line shows Mean, whiskers show ± SD. *Differences between control and vaccinated groups are statistically significant (Mann–Whitney test, p < 0.05). (E) Viral RNA presence in lungs of challenged vaccinated and control hamsters, collected on days 4, 7 and 14 post infection. Line shows Mean, whiskers show ± SD. *Differences between control and vaccinated groups are statistically significant (Mann–Whitney test, p < 0.05). (F) Viral RNA presence in visceral organs of challenged vaccinated and control hamsters, collected on days 4, 7 and 14 post infection. Numbers signify the number of positive RNA detections per total number of euthanized animals.