Abstract
BACKGROUND
Certain components of rest-activity rhythms such as greater eveningness (delayed phase), physical inactivity (blunted amplitude), and shift work (irregularity) are associated with increased risk for drug use. Dopaminergic (DA) signaling has been hypothesized to mediate the associations, though clinical evidence is lacking.
METHODS
We examined associations between rhythm components and striatal D1 (D1R) and D2/3 receptor (D2/3R) availability in 32 healthy adults (12 female, 20 male; age 42.40 ± 12.22 years) and its relationship to drug reward. Rest-activity rhythms were assessed by 1-week actigraphy combined with self-reports. [11C]NNC112 and [11C]raclopride positron emission tomography (PET) scans were conducted to measure D1R and D2/3R availability, respectively. Additionally, self-reported drug-rewarding effects of 60 mg oral methylphenidate were assessed.
RESULTS
We found that delayed rhythm was associated with higher D1R availability in caudate, which was not attributable to sleep loss or so-called social jet lag, whereas physical inactivity was associated with higher D2/3R availability in nucleus accumbens (NAc). Delayed rest-activity rhythm, higher caudate D1R, and NAc D2/3R availability were associated with greater sensitivity to the rewarding effects of methylphenidate.
CONCLUSION
These findings reveal specific components of rest-activity rhythms associated with striatal D1R, D2/3R availability, and drug-rewarding effects. Personalized interventions that target rest-activity rhythms may help prevent and treat substance use disorders.
TRIAL REGISTRATION
ClinicalTrials.gov: NCT03190954.
FUNDING
National Institute on Alcohol Abuse and Alcoholism (ZIAAA000550).
Keywords: Neuroscience
Keywords: Addiction, Molecular biology
Introduction
Circadian rhythms are approximately 24-hour periodic variations in physiological and behavioral activity. Disruption in diurnal rest-activity rhythms including timing, amplitude, and regularity have been associated with increased risk for substance use disorders (SUDs). Specifically, greater eveningness (later onset of rhythms) has been associated with a higher risk for substance use in adolescents (1–3). In adults, irregular sleep-wake schedules such as shift work increase the odds of heavy drinking (4). Physical activity and exercise, which modulate rest-activity rhythms’ amplitude (5), help prevent drug use during adolescence and adulthood (6–8). Conversely, individuals with SUDs display disrupted circadian rhythms (e.g., reduced expression of circadian clock genes, blunted and phase shifted [mostly phase delayed] circadian rhythms) (9–12). Importantly, restoring circadian rhythmicity in individuals with SUDs decreased relapse (13–15).
Dopaminergic (DA) neurotransmission, which plays an essential role in drug reward and addiction, has been hypothesized to mediate the association between circadian rhythms and addiction. Studies in laboratory animals have shown a reciprocal relationship between circadian systems and DA (16–18). Specifically, disruption of circadian systems and clock genes affects DA activity (19–21), whereas DA drives circadian gene expression (22, 23). In humans, brain functions that show associations with rest-activity phase timing, amplitude, and regularity are strongly modulated by DA (3, 24–26). So far, human studies on the associations between rest-activity rhythms and DA have focused on physical activity and exercise, and findings have been inconsistent. While structured exercise increased striatal D2/3R in 4 patients with Parkinson disease (PD) and in 19 methamphetamine users (15, 27), it decreased it in healthy adults between 63.9–77.7 years of age (28). On the other hand, self-reported intensity of habitual physical activities and baseline aerobic fitness were associated with higher striatal D2/3R availability in elderly individuals (28, 29). The conflicting findings are likely due to healthy versus clinical populations, age of participants, and differences in the radiotracers used across studies. The association between components of rest-activity rhythm and DA signaling in humans is mostly unexplored.
To address this gap, the current study aimed to examine the associations between rest-activity rhythms (height, timing, and regularity), a proxy measure of endogenous circadian rhythms (30), and striatal DA receptor (D1R and D2/3R) availability in healthy adults, and to assess how they relate to subjects’ sensitivity to the reward effect of the psychostimulant drug methylphenidate (MP). For this purpose, we assessed diurnal rest-activity and sleep-wake patterns, striatal D1R and D2/3R availability, and subjective drug effects of oral MP. Since preclinical studies have shown that higher striatal D1R and lower D2/3R enhance vulnerability to drug use (31–33), and lower D2/3R availability has been consistently reported in individuals with SUD (34–38), we hypothesized that delayed phase timing, physical inactivity, and greater irregularity of rest-activity rhythms would correlate with higher D1R, lower D2/3R availability and greater sensitivity to MP’s subjective rewarding effects in healthy adults.
Results
Diurnal measures of rest-activity rhythms.
Rest-activity and sleep-wake rhythms were assessed by 1-week actigraphy combined with self-reported sleep and exercise history during the last 6 months. For analyses of actigraphy data, both parametric measures and nonparametric measures were used (see Methods for more details). We grouped the diurnal measures into 3 categories: rhythm height, rhythm timing, and rhythm regularity.
Rhythm height parameters included parametric measures of amplitude (peak-nadir difference) and mesor (approximate middle of the modelled rhythm); nonparametric measures of means for the 5-hour period with lowest activity (L5, roughly nighttime activity level), the 10-hour period of maximum activity (M10, roughly daytime activity level), the activity across 24 hours (daily mean activity), and the M10-L5 (nonparametric amplitude); and self-reported exercise. Of note, amplitude in parametric measures (differences between a momentary peak and nadir) is conceptually different from amplitude in nonparametric measures (i.e., M10-L5, differences between means calculated over multiple hours).
Rhythm timing included parametric measures of acrophase (time of peak activity level), up-mesor (time when activity passes up through mesor, approximately the time of increasing activity in the morning), down-mesor (time when activity passes down through mesor, approximately the time of settling down for the night); nonparametric measures of sleep onset, wakeup time, sleep midpoint (time point between onset and end of sleep period time-window [SPT-window]), L5 starting time (L5hr), and M10 starting time (M10hr; ref. 39); and self-reported sleep onset, wakeup time, and chronotype.
Rhythm regularity included parametric measure of pseudo-F statistic, which was quantified by how well the obtained rest-activity data fit the 24-hour rhythm model. Lower F values indicate poorer model fit and greater rhythm irregularity. Nonparametric measures included intra-daily variability (IV) for the variations of rest-activity rhythm within each 24-hour period and inter-daily stability (IS) for the similarity of one 24-hour period to the next (40).
For representative actograms of rest-activity patterns please see Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/JCI149722DS1
Correlations between diurnal outcomes, sleep duration, and weekend-weekday (WE-WD) sleep inconsistency.
For rhythm height, self-reported exercise during the last 6 months and nonparametric measures (M10, daily mean activity, M10-L5) were significantly correlated with each other (all r > .693, all PFDR < .001). Follow-up analyses showed that these nonparametric measures were associated with self-reported exercise frequency and duration (all r > .435, all PFDR < .025) but not with self-reported subjective exercise intensity (all r < .250, all P > .05).
For rhythm timing, all parametric and nonparametric measures and self-reported sleep onset, wakeup time, and MEQ chronotype were correlated with each other (all r > .393, all PFDR < .040) except for the correlation between the up- and down-mesor measures (r = .310, P = .109).
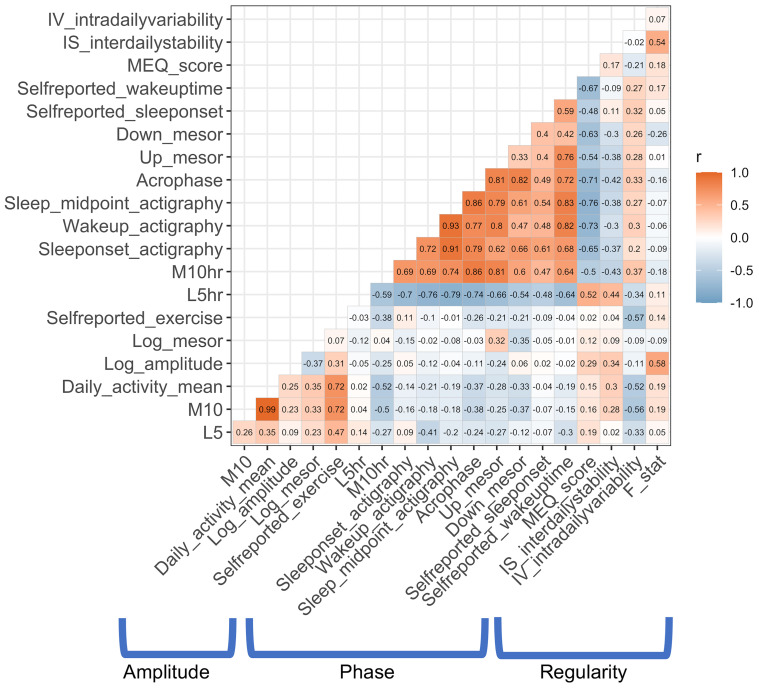
For rhythm regularity, the parametric F statistic correlated with the nonparametric IS measure (r = .535, PFDR = .012), while IV was not correlated with F or IS (all P > .131). Please see Figure 1 for intercorrelations among all diurnal measures.
Figure 1. Intercorrelations among rest-activity rhythm parameters.
Pearson’s correlation coefficients between objective and subjective measures of rest-activity rhythms used in the present study.
Longer sleep duration calculated from actigraphy data (6.98 ± 1.21 hours) was associated with later up-mesor (r = .386, P = .047), later wakeup time (r = .560, P = .002), and lower L5 (r = –.697, P < .001). None of the correlations with self-reported sleep duration were significant. Greater inconsistency for WE-WD sleep midpoint was associated with later sleep onset (r = .423, P = .025) and sleep midpoint (r = .389, P = .041). The associations between WE-WD inconsistent sleep duration and measures of rhythm timing were not significant.
Rest-activity rhythms and DA receptor availability.
As we tested for D1R and D2/3R in caudate, putamen, and NAc ROIs, we used Bonferroni’s correction to set the significance level to P < .008 (.05/6), 2-sided, for multiple comparisons. We reported the results as a trend when P > .008 and P < .05. Age, sex, and BMI were included as covariates for all analyses.
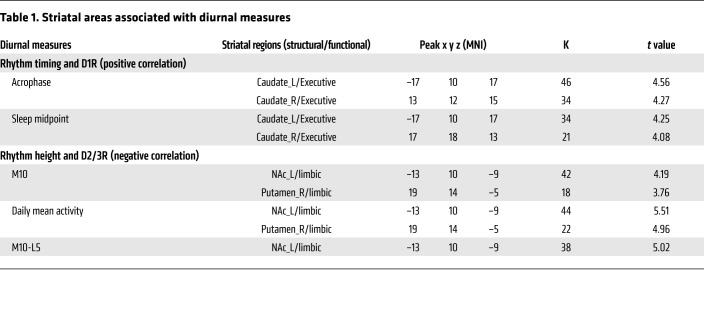
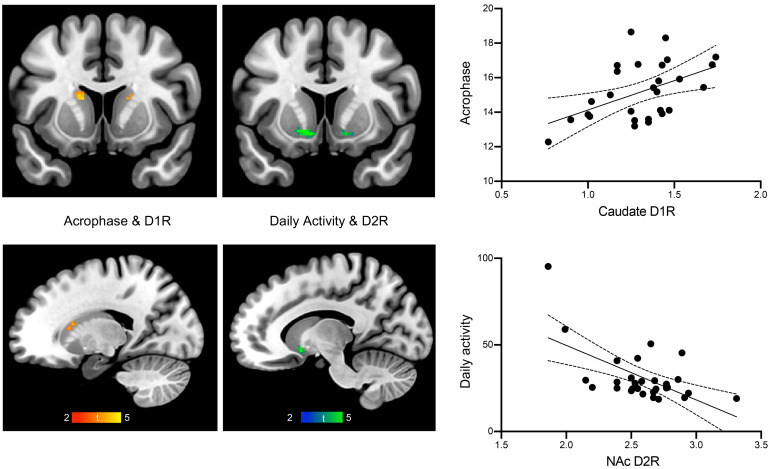
For rhythm height, M10 (r = –.564, P = .008), daily mean activity (r = –.593, P = .005), and self-reported exercise (r = –.575, P = .006) were negatively correlated with NAc D2/3R. Follow-up analyses that included race and the number of days between the PET scans and the actigraphy collection as covariates did not significantly change results (all P < .029). Further analyses revealed that greater self-reported exercise frequency (r = –.563, P = .008) and duration (r = –.502, P = .020) but not intensity (r = –.328, P = .147) were associated with lower NAc D2/3R. There were also trends for negative correlations between parametric and nonparametric rhythm amplitude (M10-L5) and NAc D2/3R; between daily mean activity and putamen D2/3R; and between M10, M10-L5, daily mean activity, and NAc D1R (all r < –.440, all P < .05 and P >.008). The voxelwise analyses corroborated these findings, which showed an association between higher M10, M10-L5, and daily mean activity and lower D2/3R availability in left NAc and right putamen (all PFWER < .05; Table 1 and Figure 2).
Table 1. Striatal areas associated with diurnal measures.
Figure 2. Rhythm components and DA receptor availability.
Timing and amplitude of rest-activity rhythms are associated with caudate D1R and NAc D2/3R, respectively. Results from voxel-wise analyses controlling for age, sex and BMI. Acrophase represents time of day with peak activity (left, middle). Zero-order correlations between rhythm components and DA receptor levels from ROI analyses (right). The solid lines indicate the lines of best fit and the dashed lines indicate the 95% confidence intervals.
For rhythm timing, acrophase (r = .575, P = .003) and down-mesor (r = .585, P = .003) were positively correlated with caudate D1R, such that later times for peak activity and for setting down for the night were associated with higher D1R. Follow-up analyses that included race and number of days between PET scans and actigraphy collection as covariates did not significantly change the results (all P < .018). There were trends for positive correlations between M10hr, sleep timing measures (objective sleep onset, sleep midpoint, self-reported wakeup time), self-reported eveningness, and caudate D1R, and between acrophase, down-mesor, M10hr, and NAc D1R (all r > .416, all P < .05 and P >.008). Since later sleep onset and sleep midpoint were correlated with a greater WE-WD inconsistent sleep midpoint, we performed follow-up analyses to determine whether their associations with caudate D1R were due to later phase or social jet lag reflected by greater differences in sleep timing between WE and WD. The associations between sleep onset (r = .659, P < .001), sleep midpoint (r = .530, P = .008), and D1R in the caudate remained significant after controlling for a WE-WD inconsistent sleep midpoint. The voxelwise analyses corroborated these findings, which revealed significant positive correlations between acrophase, sleep midpoint, and D1R availability in the caudate (all PFWER < .05; Table 1 and Figure 2).
For rhythm regularity, there was a trend for a negative correlation between F values (r = –.479, P = .018), IS (r = –.435, P = .034), and NAc D2/3R. The voxelwise analyses for the association of the measures of rhythm regularity and D2/3R availability were not significant.
Drug effects.
Delayed rhythm timing (acrophase and down-mesor) correlated with higher subjective drug rewarding effects (peak and AUC “like” and “want”) (all r > .388, all P < .041). After controlling for race, acrophase remained positively correlated with higher subjective drug rewarding effects (AUC “like” and “want”; all P < .047). No significant correlations between drug rewarding effects and other components of rest-activity rhythms were found.
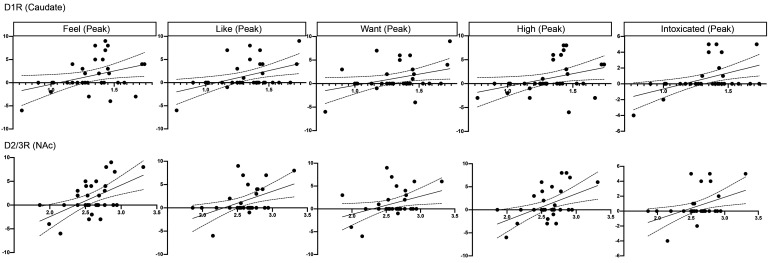
Higher caudate D1R and NAc D2/3R were associated with stronger MP-induced subjective drug effects (peak and AUC “feel,” “like,” “want,” “high,” and “intoxicated”) (all r > .357, all P < .045; Figure 3 and Supplemental Figures 2 and 3).
Figure 3. Association between DA receptor availability and subjective drug effects (peak).
The maximum drug effects (peak) were used as outcome measures. We subtracted placebo from MP ratings to estimate MP-induced subjective drug effects (y axes). D1R in the caudate (upper panel) and D2/3R in the nucleus accumbens (NAc) (lower panel) (x axes). The solid lines indicate the lines of best fit and the dashed lines indicate the 95% confidence intervals.
Covariates.
We further explored the effects of covariates (age, BMI, sex, race) on diurnal outcomes, DA receptors, and drug effects. Age was significantly correlated with advanced rhythm timing (i.e., earlier objective wakeup time [r = –.536, P = .003], advanced sleep midpoint [r = –.447, P = .015]), but not with other rhythm components. Older participants had lower D1R (caudate, putamen, NAc; all r < –.581, all P < .001), lower D2R levels (caudate, NAc; all r < –.368, all P < .038), and experienced lower MP-induced drug effects (i.e., lower self-reported “feel” and “high” [all r < –.401, P > .023]). No significant associations of sex or BMI (range: 21.2–39.5) were observed. In females, neither menopause (all t < .18, P > .863) nor menstrual cycle (all r < .679, all P > .064) had significant effects on D2/3R availability or subjective drug effects.
Black and White participants did not significantly differ in any diurnal outcomes (all t < .915, all P > .368) or subjective drug effects (all t < 1.73, all P > .095). Black participants had higher D1R in NAc than White participants (t = –2.23, P = .034). However, Black participants were younger than White participants (t = 2.39, P = .024; Black: 38.84 ± 9.61, White: 48.73 ± 12.58), which could affect DA receptor availability, rhythm phase, and subjective drug effects, as reported above. After controlling for age, the analyses showed no race differences in NAc D1R (P = .336), greater subjective drug effects (peak “want” and “high”) in White than Black participants (all marginal mean differences > 2.34, all P < .048), and no race differences in rhythm timing (all P > .143).
WE-WD sleep midpoint was not significantly associated with DA receptor availability or drug-induced rewarding effect (all P > .231). However, these findings could be affected by age, which was negatively associated with WE-WD sleep midpoint (r = –.447, P = .015). Thus, we controlled for age and found that greater WE-WD sleep midpoint was associated with lower D1R (r = –.456, P = .017) and D2R (r = –.471, P = .013) in the caudate and lower drug effects (peak “like”) (r = –.429, P = .025). The results remained unaltered after additionally controlling for the average sleep midpoint (all r < –.415, P < .035).
Discussion
The current study documents a positive association between delayed phase of rest-activity rhythms and caudate D1R availability and between physical inactivity and NAc D2/3R availability. Caudate D1R and NAc D2/3R availability were positively associated with higher self-reports of “liking,” “wanting,” and “feeling drug effects.” Additionally, delayed rhythm timing (acrophase and down-mesor) was also positively associated with higher MP-induced self-reports of drug liking and wanting. In humans, the correlation between rest-activity rhythms and drug use has been well documented. Here, we show that different rhythm components might affect the sensitivity to the rewarding effects of stimulant drugs through separate DA signaling pathways.
Delayed rhythm phase and higher D1R.
Healthy adults with later rest-activity rhythms, which were associated with higher D1R in caudate, reported higher subjective drug effects similar to previously reported findings in adolescents (1–3). Higher D1R also correlated with more intense self-reported drug effects, which is also consistent with preclinical findings that D1R levels were positively associated with greater behavioral responses to psychostimulant drugs (31, 32, 41). As hypothesized, delayed phase of rest-activity rhythms was associated with greater D1R availability in healthy adults. Preclinical studies have shown that D1R in the suprachiasmatic nucleus (SCN), the master circadian clock that controls daily activity, are involved in circadian period setting (42). In the dorsal striatum, D1R (but not D2R) can regulate internal clocks, such as feeding-related daily rhythms, independent of the SCN (43). Furthermore, systemic administration of D1R agonists can induce phase shift, whereas D1R antagonists attenuate it (44–46). The advancement of the circadian phase with melatonin administration significantly decreased alcohol intake in rats (47), though it is unclear if this effect involves D1R signaling. Prior studies reported that older adults experience advanced rest-activity rhythm (48) and have lower striatal D1R availability than younger adults (48, 49), but to our knowledge no study has reported on the association between these 2 measures in humans. We believe the current study is the first one to show evidence that in humans, circadian phase is positively associated with D1R and to provide support for a potential link between age-associated advancement in rest-activity rhythms and decreases in D1R (48, 49). Working/school schedules in subjects with greater eveningness (later sleep timing) can interfere with sleep duration and lead to inconsistent sleep between WE and WD (i.e., longer sleep duration and later sleep timing on WE known as social jet lag). It is therefore possible that the association between delayed rest-activity rhythms and increased risk of drug use might be mediated by sleep loss or inconsistent sleep, affecting cognitive control, reward processing, and decision making (24, 26, 50, 51). However, in our findings, the correlations between delayed rest-activity rhythms and greater D1R availability and the drug effect were independent of sleep loss and social jet lag. The independence of circadian rhythms and sleep homeostasis was previously supported by studies showing that they affected different brain regions in healthy adults (24, 52). As sleep deprivation downregulates D2/3R in NAc (53), the current finding suggests that delayed rest-activity rhythm and sleep loss might increase the risk of drug use through independent mechanisms. Our exploratory analyses on social jet lag demonstrated a negative relationship between WE-WD sleep timing and D1R and D2R availability in the caudate that was independent of average sleep timing and strongly modulated by age. This finding needs to be replicated in different age groups in the future and with actigraphy data over multiple weeks that could provide more reliable WE-WD measurements.
Our study included participants with a wide age range (22–64 years; mean 42 years), and thus most participants were at a life-stage of low risk for developing a SUD, compared with adolescents or younger adults who are undergoing critical developmental changes and tend to experience the greatest mismatch between delayed circadian phase and imposed school/work schedules (54–56). Thus, future studies are needed to replicate our findings in adolescents and young adults who have the greatest vulnerability to develop SUD. Although the age range of our sample might not be optimal to examine SUD risk, our findings advance the knowledge of how rest-activity patterns and DA signaling influence the sensitivity to the rewarding effects of stimulant drugs, which differ markedly among individuals.
Previous studies suggest a trend of greater morningness in Black adults (57, 58). However, we did not observe a race effect on circadian timing and our findings remained essentially unaltered after controlling for race. Interestingly, White participants reported higher subjective drug effects than Black participants without differences in DA receptor availability or rest-activity rhythms, suggesting a contribution from other mechanisms.
Blunted rhythm amplitude/physical inactivity and higher D2/3R.
Contrary to our hypothesis, blunted rest-activity rhythm, as reflected by less daily activity, M10 (daytime activity), M10-L5, and self-reported exercise history, correlated with higher D2/3R in NAc. As physical activity (M10 and daily mean activity) and rhythm amplitude (M10-L5) estimated from 1-week actigraphy data were also highly correlated with self-reported exercise history in the last 6 months, the current findings indicate that an active lifestyle in which exercise had a major contribution was associated with lower NAc D2/3R availability. In interpreting this finding, several factors should be taken into account. First, physical activity and exercise activate the mesolimbic dopamine reward pathway, as drugs of abuse do (5, 59, 60), though the duration and intensity of its activation is unclear. Our results are consistent with prior findings that the NAc, the key region of the mesolimbic circuitry, displayed the largest regional association with exercise (61, 62). Considering the neuroplasticity of the DA system, the effect of physical activity might depend on an individual’s basal DA and D2/3R levels. Indeed, in an MPTP Parkinson disease (PD) mouse model that exhibited DA depletion and lower D2R, 6 weeks of exercise increased D2R levels (63), whereas in healthy animals, 6 weeks of voluntary wheel running decreased D2R levels in the NAc (59). In rodent models of ADHD and traumatic brain injury that exhibited higher than normal striatal D2R expression, exercise inhibited D2R expression (64, 65). Similar findings were observed in humans. Using PET imaging with the radioligand [18F]fallypride, exercise-induced D2/3R increases were more pronounced in patients who had lower D2/3R and a blunted DA system such as in patients with PD (27) and in methamphetamine users (15), compared with healthy controls (27). While habitual exercise was associated with higher D2/3R availability in adults older than 63 years of age (28, 29) assessed with PET [11C]raclopride, another study of healthy subjects 23 to 80 years old showed that in the younger half of the subjects (age range 23–46 years) there was a trend for a negative correlation between physical activity and NAc D2/3R (P = .067), as assessed with PET [18F]fallypride (61). Similarly, we found that age affected the relationship between physical activity and NAc D2/3R availability such that more physical activity was correlated with lower D2/3R availability in young subjects whose age was comparable to the younger half in the previous study (61) but not in older subjects (see Supplemental Material). The findings suggest a complex relationship between physical activity and D2/3R at different stages of adulthood that might relate to age-related DA and D2R decline (66) and to different baseline levels of physical activity between young and old adults. Second, duration, frequency, and intensity of exercise might differ in their correlation with D2/3R in dorsal and ventral striatum. In our study, self-reported duration and frequency (but not intensity) of exercise in the last 6 months were correlated with lower D2/3R in NAc and ventral putamen. Previous studies observed a positive correlation between self-rated intensity (but not frequency) of habitual physical activity and objective aerobic fitness and D2/3R availability in caudate in adults older than 63 years of age (28, 29). Moreover, future studies that assess heart rate as an objective measure of exercise intensity will allow researchers to test whether the association with D2/3R differs between objective and subjective measures (67). Finally, the mixed findings across studies could be due to the different PET ligands used to measure D2/3R. Exercise by downregulating DAT (68, 69) and nigral D2-autoreceptors (70) can induce continuous high basal DA levels. We used [11C]raclopride, which is a ligand with a higher sensitivity to competition with endogenous DA for binding to D2/3R in the striatum (71–73) than higher-affinity ligands such [18F]fallypride (73, 74), hence our measures are likely to be more heavily influenced by DA than studies that used [18F]fallypride (15, 27). With [11C]raclopride we cannot distinguish whether the inverse association of exercise with D2/3R reflects higher baseline extracellular DA levels or lower levels of D2/3R in physically active participants.
In addition, there is evidence that D2R signaling can modulate circadian amplitude. Stimulating D2R by endogenous DA or by D2R agonists reduced circadian rhythm amplitude and suppressed clock gene expression in laboratory animals (23, 75). In humans, low doses of D2/3R agonists induce sleepiness in healthy controls (76), presumably through stimulation of autoreceptors, whereas higher doses increased locomotor activity (77), presumably through stimulation of postsynaptic receptors.
As it relates to drug effects, chronic exercise diminished amphetamine-induced striatal DA release in laboratory animals (78). Though in our study, we did not see an association between physical activity and lower subjective drug effects, participants with lower NAc D2/3R, which correlated with greater physical activity, had lower subjective drug effects when given MP. In animals, lack of D2R decreases sensitivity to drugs (79, 80). Clinical studies in SUDs have consistently reported reduced striatal D2/3R availability (34–38). However, its correlation with behavioral effects varied across different types of drugs (36–38, 81). While lower D2/3R predicted relapse in methamphetamine users (36) and was associated with addiction severity in marijuana abusers (37), no correlations were found in cocaine or heroin users (38, 81). In nonaddicted healthy adults, low baseline D2/3R was associated with high sensitivity to the rewarding effects of i.v. MP (0.5 mg/Kg; refs. 82, 83), whereas it was correlated with low subjective response to i.v. alcohol (84) and showed no correlation with behavioral response to i.v. amphetamine (0.3 mg/kg; ref. 85). The discrepancy among findings could result from pharmacological differences between drugs, doses given, and route of administration (i.v. vs. oral). Drugs of abuse exert their reinforcing effect by increasing DA transmission, though the magnitude of DA increases and the pharmacological mechanisms by which they do it differ among drugs. For example, alcohol increases DA release primarily by enhancing DA cell spiking activity, whereas psychostimulants such as MP and amphetamine increase DA transmission by blocking DA reuptake and by releasing DA from the synapse, respectively, leading to much higher levels of extracellular DA (e.g., 35-fold) compared with alcohol (2-fold; ref. 86). Furthermore, i.v. administration of stimulant drugs induces faster and larger rewarding effects (82, 83) than oral administration, which takes around 50 minutes to achieve its effect (Supplemental Figure 4). Different magnitude and dynamics of DA increases can subsequently influence stimulation of D1R and D2/3R, which differ in their affinity for DA (87).
Finally, D1R and D2/3R signaling associated with rest-activity rhythm components were region-specific (Figure 2). The association of rhythm timing and D1R was predominantly with caudate (region engaged in executive processes) and that of physical activity and D2/D3R was predominantly with NAc (region engaged with reward and motivation; refs. 88–91), which raises the question of how distinct components of rest-activity rhythms impact brain functions (92) and merits further investigation.
Effects of the time of day on the scans and MP administration.
PET scans and MP administration were conducted at the same time of the day for all participants. Specifically, the [11C]NNC112 scans were done at 10 am, the administrations of MP were done at 12 pm, and the [11C]raclopride scans were done at 1 pm. There is evidence that time-of-day affects the sensitivity to rewards and the craving for drugs of abuse in both laboratory animals and humans (93–95). Interestingly, a recent study reported that people with later sleep timing had later timing of peak craving for alcohol and the differences in alcohol craving between people with earlier timing and later timing were greatest in the early morning (around 6–8 am) and evening (around 5–8 pm; ref. 94). Therefore, it is possible that the association we observed between acrophase and the subjective rewarding effects of MP, which was given at 12 pm, might vary across the time of day.
The influence of time-of-day on the sensitivity to rewards might be mediated by DA, as diurnal variation of DA signaling has been demonstrated within the striatum in animal models (96, 97). DA uptake through DAT rather than DA metabolism, D2R autoreceptor inhibition, or DA neuron firing seems to account for diurnal variations in extracellular DA (98, 99). In rats, D1R availability is stable across the 24-hour period (100). In humans, there are no significant differences in D2R availability between night and daytime for both healthy controls and patients with restless-legs syndrome (101). Together, although extracellular DA has a circadian profile, DA receptor availability levels seem stable across the time of day. The current findings of associations between rest-activity rhythms and DA receptor availability are less likely to be influenced by the time of day when the scans were performed.
Clinical implications.
The current study identifies the potential value for the development of personalized interventions targeting rest-activity rhythms to prevent and treat SUDs. Shifting the phase of rest-activity rhythms might help modify DA signaling and the function of brain reward regions and have add-on benefits to that of sleep interventions, which only target sleep duration. Given the seeming independence between circadian/diurnal rhythms and sleep homeostasis (24, 52) and that sleep affects numerous mechanisms beyond those modulated by DA, such as clearance of β-amyloid from the human brain (102), interventions combining circadian and sleep components would maximize their beneficial effect on rewarding and cognitive function, including potential protective effects for neurodegenerative diseases such as Alzheimer’s disease (103). As reviewed and discussed in Lynch et al. (17), the beneficial effects of physical activity on drug addiction likely depend on basal DA levels and on the intensity, duration, and frequency of exercise, so interventions should be personalized to each individual and to the different addiction stages. Furthermore, the current findings indicate that timing and amplitude of rest-activity rhythms might affect sensitivity to drug reward through different DA pathways. Interventions that combine these 2 rhythm factors such as exercise shortly after wakeup might help accelerate recovery in SUDs more than interventions that focus on only one factor.
Limitations.
Our study identified association between rest-activity rhythms and DA receptor availability, but it cannot establish causality, which will require intervention studies to manipulate rest-activity rhythms and evaluate its effects on DA receptor availability or vice versa. Also, wrist activity alone cannot be used as pure markers of SCN circadian output. Although rest-activity rhythms measured by actigraphy are strongly influenced by SCN signaling, they are susceptible to masking effects from imposed social schedules (30) so we cannot infer with certainty that our findings in rest-activity rhythms are due to endogenous circadian rhythms. Inclusion of circadian measures, such as melatonin and core body temperature, to assess their association with DA signaling would be an important next step. The sample size is another limitation, which might have affected our power to detect associations between DA and rhythm irregularity as indirectly documented by fMRI studies (24, 26) and to evaluate whether there are race and sex differences in the association with rest-activity rhythms, DA signaling, and rewarding effect of drug use (57, 58, 104). Indeed, the current study observed a medium (r > .4) negative correlation between NAc D2/3R and rhythm regularity that did not survive correction for multiple comparison, and this might be attributed to the limited sample size. Additional limitations are caused by the imperfect selectivity of [11C]raclopride, which binds to both pre- and postsynaptic D2R and D3R and has similar affinity for these 2 receptors. Thus, our measurements reflect a combination of signals from pre- and postsynaptic D2Rs and D3Rs, which are distinctly affected by exercise and physical activity. In laboratory animals, exercise reduces presynaptic D2 autoreceptors and increases postsynaptic D2R (5, 70), whereas the lack of postsynaptic D2R blunts motor responses and the lack of presynaptic D2 autoreceptors induces hyperactivity (105–107). The balance between pre- and postsynaptic D2R in different striatal regions is also critical as their regulations of DA are region-specific (107). Furthermore, as D3R has higher affinity than D2R for DA, endogenous DA will interfere with [11C]raclopride binding to D3R more than for D2R (108, 109). Since the distribution of D3R is higher in NAc than in dorsal striatum (110, 111), D2/3R binding measurements in NAc will be affected by DA more than in dorsal striatum. This is also relevant since D3R signaling can directly influence rest-activity rhythms (112).
Conclusions.
The current study provides what we believe is novel evidence for a direct correlation between different components of rest-activity rhythms and striatal D1R and D2/3R availability that were associated with sensitivity to the rewarding effects of the stimulant drug MP in healthy adults. Personalized interventions that target rest-activity rhythms might help as strategies to prevent and treat SUDs.
Methods
Participants.
Thirty-two participants (12 female, 20 male; age 42.40 ± 12.22 years, range: 22–64 years; 46.9% Black, 43.8% White, 6.3% Asian, 3.1% other races) completed the study. Participants were recruited through referrals from the NIH Volunteer Office, ResearchMatch.org, and IRB-approved advertisements. Data were collected at National Institute on Alcohol Abuse and Alcoholism from 2017–2019. Exclusion criteria were history of substance abuse or dependence other than nicotine (n = 1 self-reported smoker with Fagerstrom test for nicotine dependence [FTND] score = 0) or current or past history of psychiatric disorder, neurological disease, medical conditions that may alter cerebral function (i.e., cardiovascular, endocrinological, oncological, or autoimmune diseases), current use of prescribed or over-the-counter medications, and/or head trauma with loss of consciousness longer than 30 minutes.
Objective rest-activity/sleep-wake measures.
To record rest-activity/sleep-wake patterns, participants wore a GENEActiv triaxial accelerometer (version 1.1, Activinsights Ltd) placed on the nondominant wrist continuously for 1 week. Thirty participants had valid actigraphy data. The number of days between [11C]NNC112 scan and actigraphy collection was –9.27 ± 63.64 days and between [11C]raclopride scan and actigraphy collection was –17.67 ± 65.21 days, which were controlled for in the follow-up analyses. Negative values denote actigraphy collection started before the PET scans. For actigraphy analyses, we applied both parametric and nonparametric measures as they are complementary (113). While parametric measures capture the size, timing, and shape of rhythms (114), nonparametric measures are based on raw data counts and thus do not rely on a priori assumptions about the waveform (e.g., a cosine shape of activity data) (115).
For parametric analysis, acceleration data were averaged within 60-second epochs using GENEActiv PC Software 3.0. A sigmoidally transformed extended cosine model adapted from Marler et al. (114) was applied to fit the rest-activity data using R package RAR (version 2.0.0).
For nonparametric analysis, we used the R package GGIR (version 2.2-0) to process the raw accelerometer data in .bin format. Procedures for the identification of SPT-window by GGIR were as previously described (116).
Self-reported sleep and exercise history.
Participants reported their sleep (bedtime, wakeup time) and exercise history (frequency, duration, and intensity) during the last 6 months. An index of exercise was calculated as follows: frequency (per week) × duration × intensity. Chronotype was assessed with morningness-eveningness questionnaire (MEQ; ref. 117).
MRI scanning.
A T1 (3D MP-RAGE; TR/TE = 2400/2.24 ms) and T2 (SPACE; TR/TE = 3200/564 ms) image, each with 0.8 mm isotropic voxels, were acquired on a 3.0T Magnetom Prisma scanner (Siemens Medical Solutions USA) with a 32-channel head coil. The minimal preprocessing pipelines of the Human Connectome Project (118) and FreeSurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu) were used for spatial normalization to the stereotactic MNI space of the structural scans and to segment the anatomical images and obtain masks for the NAc, caudate, putamen, and cerebellum regions of interest (ROIs; ref. 119).
PET scanning.
PET scans for each participant were performed on 1 of 2 available scanners: a high-resolution research tomography (HRRT) scanner (n = 16; 7 female, 9 male; Siemens AG) or a Biograph PET/CT scanner (n = 16; 5 female, 11 male; Siemens AG). The methods for correcting differences between scanners are described below. PET and diurnal measures did not differ among participants using different scanners (Supplemental Table 1). A venous catheter was placed in the antecubital vein for radiotracer injection. After the patient was positioned in the scanner, a transmission scan was obtained to correct emission images for attenuation. Emission scans were obtained using 3D list mode and were started immediately after tracer injection (1-minute bolus). During scanning, subjects rested quietly under dim illumination and with minimal acoustic noise. To ensure subjects did not fall asleep, they were monitored throughout the procedure and were asked to keep their eyes open. Dynamic emission scan images were evaluated before analyses to ensure that motion artifacts or misplacements were not included.
For each participant, one D1R scan, for which emission data were collected for 90 minutes after a maximum injection of 15 mCi [11C]NNC112 (specific activity 4543.71 ± 2637.10 mCi/μmol; ref. 120), and one D2/3R scan, for which emission data were collected for 60 minutes after a maximum injection of 10 mCi [11C]raclopride per scan (specific activity = 5178.77 ± 2864.40 mCi/μmol; ref. 110) were conducted.
The [11C]NNC112 scan was performed (10 AM) either before the [11C]raclopride scan (1PM) on the same day or on 2 separate days. Both scans were conducted in the same scanner for a given subject.
PET analysis.
Dynamic PET images were coregistered to high-resolution MRI scans in subject space, transformed to MNI space with the Human Connectome Project normalization parameters, and resampled to 2-mm isotropic resolution (using Analysis of Functional NeuroImages [ref. 121] and FMRIB Software Library functions [ref. 122]). Differences in geometry and point spread function (PSF) between cameras (PET/CT = 4 mm PSF; HRRT = 2.7 mm PSF) originated systematic voxelwise differences in signal intensity between PET/CT and HRRT images. To correct for scanner-specific scaling effects and harmonize the data, we used a voxelwise approach based on grand-mean scaling. Specifically, the scaling matrix M(x,y,z) was estimated from m = 16 PET/CT images, βi(x,y,z), and n = 16 HRRT images, αi(x,y,z), in MNI space using the following formula:
(Equation 1)
Successively, the PET/CT images in MNI space were corrected to match the average distribution of signal intensity of the HRRT images as follows: βci(x,y,z) = M(x,y,z) × βi(x,y,z). Please see Supplemental Figure 5 for the accuracy of our corrections for scanner differences demonstrated with phantom data.
Additionally, as a second approach to control for scanner differences, we used the original uncorrected image data and included the PET scanner as a covariate in the analyses. All findings remained essentially unaltered (Supplemental Table 2).
The standardized uptake value–based (SUV-based) reference tissue method was used to calculate an SUV ratio (SUVr) between a target region and the cerebellar cortex, used as reference region, which was used to quantify receptor availability in caudate, putamen, and NAc ROIs.
Subjective drug effects.
To assess subjective drug effects, participants were questioned by an experimenter using a drug effects questionnaire (DEQ) that included the following questions: (a) “Do you feel any drug effects?” (feel), (b) “Do you like the effects that you are feeling right now?” (like), (c) “Would you like more of what you received right now?”(want), (d) “Do you feel high?” (high), and (e) “Do you feel intoxicated?” (intoxicated). Questions were rated on a scale of 1 to 10, with 1 being not at all and 10 being maximum, and obtained at 5 minute intervals, starting 5 minutes (at 12 pm) before drug administration (baseline) until 2 hours after administration of oral placebo or 60 mg oral MP pill (double-blinded, assessed on 2 separate days). The AUC of the changes from baseline and the maximum drug effects (peak) were used as outcome measures. We subtracted placebo from MP ratings to estimate MP-induced subjective drug effects.
Statistics.
Analyses were performed in SPSS Statistics Subscription (IBM). Pearson’s correlations were used to calculate the intercorrelation among various diurnal measures of rest-activity rhythms. As sleep might interfere with rest-activity rhythm and affect the results and interpretation, we examined the correlations between the rest-activity rhythm and sleep duration and sleep differences between WE and WD, (i.e., WE-WD sleep duration and WE-WD sleep midpoint), referred to as social jet lag induced by working/social schedules. We also explored the relationship between WE-WD sleep and DA receptor availability and drug effects. Partial correlations were performed for associations between diurnal measures and D1R and D2/3R availability in striatal ROIs, controlling for age, sex, and BMI. As follow-up analyses, we additionally controlled for sleep if it was significantly correlated with diurnal measures, and included the number of days between PET scans and actigraphy collection as a covariate. As we tested for D1R and D2/3R in caudate, putamen, and NAc ROIs, we used Bonferroni’s correction to set significance level to P < .008 (.05/6), 2-sided, for multiple comparisons. When significant, we tested for their (diurnal outcomes and DA receptor availability) correlations with MP-induced subjective drug effects. Also, we explored the effects of covariates (age, BMI, and sex) on diurnal outcomes, DA receptor availability, and drug effects using Pearson’s correlations and independent t test. For females, we further explored the potential effect of menses and menstrual cycle on D2/3R availability and subjective drug effects (123–125). We compared females who were still menstruating (n = 8) with females who were menopausal (n = 4), and examined whether the days between last menses and [11C]raclopride scan (range: 3–30 days) were associated with D2/3R availability and subjective drug effects. Previous studies have shown that Black adults tend to have shorter circadian periods and greater odds of being morning type than White adults (57, 58), so we further examined whether diurnal outcomes differed between Black and White participants using independent t test. We explored potential race differences in DA receptor availability and subjective drug effects in Black and White participants. Finally, we conducted follow-up analyses and included race as a covariate.
Since ROI analyses are limited in detecting effects in preselected anatomical regions that might be heterogenous, we also conducted voxel-based analyses on the SUVr images using SPM 8 and a general linear model (Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/) to corroborate the association between rest-activity rhythms and DA receptor availability. Diurnal measures of rest-activity rhythms were included as variables of interest, while age, sex, and BMI were included as covariates. A striatal mask composed of the bilateral caudate, putamen, and NAc ROIs was applied. Statistical significance was defined at PFWER < .05 using a cluster-level family-wise error (FWE) correction with a cluster-defining threshold of P < .001.
Study approval.
Participants provided written informed consent to participate in the study, which was approved by the Institutional Review Board at the National Institutes of Health.
Author contributions
RZ and NDV designed the study. RZ, PM, DT, ESK, DSK, DEF, KLM, CLB, and GJW conducted experiments and acquired data. RZ, PM, DT, SWK, ESK, DSK, and SBD analyzed data and performed statistical analysis. RZ and NDV drafted the manuscript. All authors contributed intellectually and approved the manuscript.
Supplementary Material
Acknowledgments
We thank Michele Vera-Yonga, Veronica Ramirez, Jamie Burns, Christopher Kure Liu, Karen Torres, Christopher Wong, Amna Zehra, Lori Talagala, and Minoo McFarland for their contributions. We thank Craig Barker for assisting with the phantom data for the revision. This work was supported by the National Institute on Alcohol Abuse and Alcoholism, NIH (ZIAAA000550).
Version 1. 07/15/2021
In-Press Preview
Version 2. 09/15/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Role of funding source: The funders had no role in study design, data collection, data analysis, or preparation of the manuscript.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(18):e149722.https://doi.org/10.1172/JCI149722.
See the related Commentary at Delayed circadian rhythms and substance abuse: dopamine transmission’s time has come.
Contributor Information
Rui Zhang, Email: rui.zhang@nih.gov.
Peter Manza, Email: peter.manza@nih.gov.
Dardo Tomasi, Email: Dardo.tomasi@nih.gov.
Sung Won Kim, Email: kims8@mail.nih.gov.
Ehsan Shokri-Kojori, Email: ehsan.shokrikojori@nih.gov.
Sukru B. Demiral, Email: sukru.demiral@nih.gov.
Danielle S. Kroll, Email: danikroll523@gmail.com.
Dana E. Feldman, Email: dfeld96@gmail.com.
Katherine L. McPherson, Email: katiemcpherson97@gmail.com.
Catherine L. Biesecker, Email: clbiesecker@email.wm.edu.
Gene-Jack Wang, Email: gene-jack.wang@nih.gov.
Nora D. Volkow, Email: nvolkow@nida.nih.gov.
References
- 1.Hasler BP, et al. Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: initial findings from the NCANDA study. Alcohol Clin Exp Res. 2017;41(6):1154–1165. doi: 10.1111/acer.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasler BP, et al. Eveningness among late adolescent males predicts neural reactivity to reward and alcohol dependence two years later. Behav Brain Res. 2017;327:112–120. doi: 10.1016/j.bbr.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasler BP, et al. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. 2013;214(3):357–364. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morikawa Y, et al. Correlation between shift-work-related sleep problems and heavy drinking in Japanese male factory workers. Alcohol Alcohol. 2013;48(2):202–206. doi: 10.1093/alcalc/ags128. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood BN. The role of dopamine in overcoming aversion with exercise. Brain Res. 2019;1713:102–108. doi: 10.1016/j.brainres.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Aaron DJ, et al. Physical activity and the initiation of high-risk health behaviors in adolescents. Med Sci Sports Exerc. 1995;27(12):1639–1645. [PubMed] [Google Scholar]
- 7.Terry-McElrath YM, O’Malley PM. Substance use and exercise participation among young adults: parallel trajectories in a national cohort-sequential study. Addiction. 2011;106(10):1855–1865. doi: 10.1111/j.1360-0443.2011.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson MC, Gordon-Larsen P. Physical activity and sedentary behavior patterns are associated with selected adolescent health risk behaviors. Pediatrics. 2006;117(4):1281–1290. doi: 10.1542/peds.2005-1692. [DOI] [PubMed] [Google Scholar]
- 9.Conroy DA, et al. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiol Int. 2012;29(1):35–42. doi: 10.3109/07420528.2011.636852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facchinetti F, et al. Hypothalamus-pituitary-adrenal axis of heroin addicts. Drug Alcohol Depend. 1985;15(4):361–366. doi: 10.1016/0376-8716(85)90014-6. [DOI] [PubMed] [Google Scholar]
- 11.Huang M-C, et al. Reduced expression of circadian clock genes in male alcoholic patients. Alcohol Clin Exp Res. 2010;34(11):1899–1904. doi: 10.1111/j.1530-0277.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiers CE, et al. Reduced sleep duration mediates decreases in striatal D2/D3 receptor availability in cocaine abusers. Transl Psychiatry. 2016;6:e752. doi: 10.1038/tp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulick D, Gamsby JJ. Racing the clock: the role of circadian rhythmicity in addiction across the lifespan. Pharmacol Ther. 2018;188:124–139. doi: 10.1016/j.pharmthera.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Morais APD, et al. The neurobiological mechanisms of physical exercise in methamphetamine addiction. CNS Neurosci Ther. 2018;24(2):85–97. doi: 10.1111/cns.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson CL, et al. Effect of exercise training on striatal dopamine D2/D3 receptors in methamphetamine users during behavioral treatment. Neuropsychopharmacology. 2016;41(6):1629–1636. doi: 10.1038/npp.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logan RW, et al. Circadian rhythms and addiction: mechanistic insights and future directions. Behav Neurosci. 2014;128(3):387–412. doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch WJ, et al. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37(8):1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClung CA. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. ScientificWorldJournal. 2007;7:194–202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garmabi B, et al. Effect of circadian rhythm disturbance on morphine preference and addiction in male rats: Involvement of period genes and dopamine D1 receptor. Neuroscience. 2016;322:104–114. doi: 10.1016/j.neuroscience.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 20.McClung CA, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102(26):9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parekh PK, et al. Circadian clock genes: effects on dopamine, reward and addiction. Alcohol. 2015;49(4):341–349. doi: 10.1016/j.alcohol.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood S, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30(42):14046–14058. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imbesi M, et al. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience. 2009;158(2):537–544. doi: 10.1016/j.neuroscience.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, et al. Sleep inconsistency between weekends and weekdays is associated with changes in brain function during task and rest. Sleep. 2020;43(10):zsaa076. doi: 10.1093/sleep/zsaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valkenborghs SR, et al. The impact of physical activity on brain structure and function in youth: a systematic review. Pediatrics. 2019;144(4):e20184032. doi: 10.1542/peds.2018-4032. [DOI] [PubMed] [Google Scholar]
- 26.Hasler BP, et al. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol Psychol. 2012;91(3):334–341. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher BE, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport. 2013;24(10):509–514. doi: 10.1097/WNR.0b013e328361dc13. [DOI] [PubMed] [Google Scholar]
- 28.Jonasson LS, et al. Higher striatal D2-receptor availability in aerobically fit older adults but non-selective intervention effects after aerobic versus resistance training. Neuroimage. 2019;202:116044. doi: 10.1016/j.neuroimage.2019.116044. [DOI] [PubMed] [Google Scholar]
- 29.Köhncke Y, et al. Self-rated intensity of habitual physical activities is positively associated with dopamine D2/3 receptor availability and cognition. Neuroimage. 2018;181:605–616. doi: 10.1016/j.neuroimage.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 30.Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 31.Caine SB, et al. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27(48):13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Probing the role of the dopamine D1 receptor in psychostimulant addiction. Ann N Y Acad Sci. 2000;914:13–21. doi: 10.1111/j.1749-6632.2000.tb05179.x. [DOI] [PubMed] [Google Scholar]
- 33.Park K, et al. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J Neurosci. 2013;33(40):15827–15836. doi: 10.1523/JNEUROSCI.1935-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 35.Volkow ND, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang GJ, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17(9):918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci U S A. 2014;111(30):E3149–E3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez D, et al. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71(3):192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quante M, et al. Zeitgebers and their association with rest-activity patterns. Chronobiol Int. 2019;36(2):203–213. doi: 10.1080/07420528.2018.1527347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witting W, et al. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 41.Xu M, et al. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852(1):198–207. doi: 10.1016/S0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 42.Smyllie NJ, et al. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 2016;113(13):3657–3662. doi: 10.1073/pnas.1511351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallardo CM, et al. Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. Elife. 2014;3:e03781. doi: 10.7554/eLife.03781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorelli CJ, et al. Resilience of circadian pacemaker development in hamsters. J Biol Rhythms. 2011;26(3):221–229. doi: 10.1177/0748730411402633. [DOI] [PubMed] [Google Scholar]
- 45.Ruan G-X, et al. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6(10):e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viswanathan N, et al. Entrainment of the fetal hamster circadian pacemaker by prenatal injections of the dopamine agonist SKF 38393. J Neurosci. 1994;14(9):5393–5398. doi: 10.1523/JNEUROSCI.14-09-05393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vengeliene V, et al. Activation of melatonin receptors reduces relapse-like alcohol consumption. Neuropsychopharmacology. 2015;40(13):2897–2906. doi: 10.1038/npp.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JH, Duffy JF. Circadian rhythm sleep-wake disorders in older adults. Sleep Med Clin. 2018;13(1):39–50. doi: 10.1016/j.jsmc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Karrer TM, et al. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 2017;57:36–46. doi: 10.1016/j.neurobiolaging.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, et al. Sleep deprivation promotes habitual control over goal-directed control: behavioral and neuroimaging evidence. J Neurosci. 2017;37(49):11979–11992. doi: 10.1523/JNEUROSCI.1612-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krause AJ, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18(7):404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muto V, et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353(6300):687–690. doi: 10.1126/science.aad2993. [DOI] [PubMed] [Google Scholar]
- 53.Volkow ND, et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32(19):6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rioux C, et al. Age of cannabis use onset and adult drug abuse symptoms: a prospective study of common risk factors and indirect effects. Can J Psychiatry. 2018;63(7):457–464. doi: 10.1177/0706743718760289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85(1):41–47. doi: 10.2105/AJPH.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roenneberg T, et al. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 57.Crowley SJ, Eastman CI. Free-running circadian period in adolescents and adults. J Sleep Res. 2018;27(5):e12678. doi: 10.1111/jsr.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malone SK, et al. Differences in morning-evening type and sleep duration between Black and White adults: Results from a propensity-matched UK Biobank sample. Chronobiol Int. 2017;34(6):740–752. doi: 10.1080/07420528.2017.1317639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenwood BN, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zlebnik NE, et al. Chronic wheel running affects cocaine-induced c-Fos expression in brain reward areas in rats. Behav Brain Res. 2014;261:71–78. doi: 10.1016/j.bbr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dang LC, et al. Reduced effects of age on dopamine D2 receptor levels in physically active adults. Neuroimage. 2017;148:123–129. doi: 10.1016/j.neuroimage.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmad SO, et al. Effects of endurance exercise on ventral tegmental area neurons in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid-treated mice. Neurosci Lett. 2009;450(2):102–105. doi: 10.1016/j.neulet.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 63.Vučković MG, et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: in vivo imaging with [18F]fallypride. Mov Disord. 2010;25(16):2777–2784. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho HS, et al. Effect of exercise on hyperactivity, impulsivity and dopamine D2 receptor expression in the substantia nigra and striatum of spontaneous hypertensive rats. J Exerc Nutrition Biochem. 2014;18(4):379–384. doi: 10.5717/jenb.2014.18.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ko IG, et al. Treadmill exercise improves memory by up-regulating dopamine and down-regulating D2 dopamine receptor in traumatic brain injury rats. J Exerc Rehabil. 2019;15(4):504–511. doi: 10.12965/jer.1938316.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rinne JO, et al. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13(2):310–314. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- 67. Nobel M, et al, eds. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018. [Google Scholar]
- 68.Fisher BE, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 69.Petzinger GM, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27(20):5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10(2):67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- 71.Hietala J, et al. Measurement of striatal D2 dopamine receptor density and affinity with [11C]-raclopride in vivo: a test-retest analysis. J Cereb Blood Flow Metab. 1999;19(2):210–217. doi: 10.1097/00004647-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Verhoeff NPLG, et al. Dopamine depletion results in increased neostriatal D 2, but not D 1, receptor binding in humans. Molecular Psychiatry. 2002;7(3):322–328. doi: 10.1038/sj.mp.4001057. [DOI] [PubMed] [Google Scholar]
- 73.Slifstein M, et al. Striatal and extrastriatal dopamine release measured with PET and [18F]fallypride. Synapse. 2010;64(5):350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cropley VL, et al. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62(6):399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- 75.Wei H, et al. Dopamine D2 receptor signaling modulates pancreatic beta cell circadian rhythms. Psychoneuroendocrinology. 2020;113:104551. doi: 10.1016/j.psyneuen.2019.104551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferreira JJ, et al. Effect of ropinirole on sleep onset: a randomized, placebo-controlled study in healthy volunteers. Neurology. 2002;58(3):460–462. doi: 10.1212/WNL.58.3.460. [DOI] [PubMed] [Google Scholar]
- 77.Monti JM, et al. Biphasic effects of dopamine D-2 receptor agonists on sleep and wakefulness in the rat. Psychopharmacology (Berl) 1988;95(3):395–400. doi: 10.1007/BF00181955. [DOI] [PubMed] [Google Scholar]
- 78.Marques E, et al. Influence of chronic exercise on the amphetamine-induced dopamine release and neurodegeneration in the striatum of the rat. Ann N Y Acad Sci. 2008;1139:222–231. doi: 10.1196/annals.1432.041. [DOI] [PubMed] [Google Scholar]
- 79.Maldonado R, et al. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388(6642):586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 80.Phillips TJ, et al. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1(7):610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 81.Martinez D, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- 82.Volkow ND, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156(9):1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 83.Volkow ND, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46(2):79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 84.Yoder KK, et al. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29(6):965–970. doi: 10.1097/01.ALC.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- 85.Martinez D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 86.Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95 Suppl 2:S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- 87.Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- 88.Cools R, et al. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41(11):1431–1441. doi: 10.1016/S0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 89.Cools R, et al. Enhanced or impaired cognitive function in Parkinson’s Disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 90.de Boer L, et al. Dorsal striatal dopamine D1 receptor availability predicts an instrumental bias in action learning. Proc Natl Acad Sci U S A. 2019;116(1):261–270. doi: 10.1073/pnas.1816704116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rieckmann A, et al. Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J Neurosci. 2011;31(40):14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tziortzi AC, et al. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex. 2014;24(5):1165–1177. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Byrne JEM, Murray G. Diurnal rhythms in psychological reward functioning in healthy young men: ‘Wanting’, liking, and learning. Chronobiol Int. 2017;34(2):287–295. doi: 10.1080/07420528.2016.1272607. [DOI] [PubMed] [Google Scholar]
- 94.Hisler GC, et al. Is there a 24-hour rhythm in alcohol craving and does it vary by sleep/circadian timing? Chronobiol Int. 2021;38(1):109–121. doi: 10.1080/07420528.2020.1838532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Keith DR, et al. Time of day influences the voluntary intake and behavioral response to methamphetamine and food reward. Pharmacol Biochem Behav. 2013;110:117–126. doi: 10.1016/j.pbb.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castañeda TR, et al. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36(3):177–185. doi: 10.1046/j.1600-079X.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 97.Paulson PE, Robinson TE. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav Neurosci. 1994;108(3):624–635. doi: 10.1037/0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- 98.Ferris MJ, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A. 2014;111(26):E2751–E2759. doi: 10.1073/pnas.1407935111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sleipness EP, et al. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 2007;1129(1):34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 100.Oskamp A, et al. Neurotransmitter receptor availability in the rat brain is constant in a 24 hour-period. Chronobiol Int. 2017;34(7):866–875. doi: 10.1080/07420528.2017.1325370. [DOI] [PubMed] [Google Scholar]
- 101.Earley CJ, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36(1):51–57. doi: 10.5665/sleep.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shokri-Kojori E, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115(17):4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nobili A, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun. 2017;8:14727. doi: 10.1038/ncomms14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hasler BP, et al. Preliminary evidence that real world sleep timing and duration are associated with laboratory-assessed alcohol response. Alcohol Clin Exp Res. 2019;43(7):1575–1584. doi: 10.1111/acer.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bello EP, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D 2 autoreceptors. Nat Neurosci. 2011;14(8):1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anzalone A, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49(3):231–252. [PubMed] [Google Scholar]
- 109.Sokoloff P, et al. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 110.Mawlawi O, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 111.Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20(1):60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 112.Yamada N, Martin-Iverson MT. Selective dopamine D1 and D2 agonists independently affect different components of the free-running circadian rhythm of locomotor activity in rats. Brain Res. 1991;538(2):310–312. doi: 10.1016/0006-8993(91)90445-2. [DOI] [PubMed] [Google Scholar]
- 113.Krafty RT, et al. Measuring variability in rest-activity rhythms from actigraphy with application to characterizing symptoms of depression. Stat Biosci. 2019;11(2):314–333. doi: 10.1007/s12561-018-09230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marler MR, et al. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 115.van Someren EJW, et al. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry. 1996;40(4):259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 116.van Hees VT, et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep. 2018;8(1):12975. doi: 10.1038/s41598-018-31266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 118.Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 120.Abi-Dargham A, et al. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20(2):225–243. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 121.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 122.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 123.Petersen N, et al. Striatal dopamine D 2 -type receptor availability and peripheral 17 β -estradiol. Mol Psychiatry. doi: 10.1038/s41380-020-01000-1. [published online January 8, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Czoty PW, et al. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34(3):548–554. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- 125.Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84(1):1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.