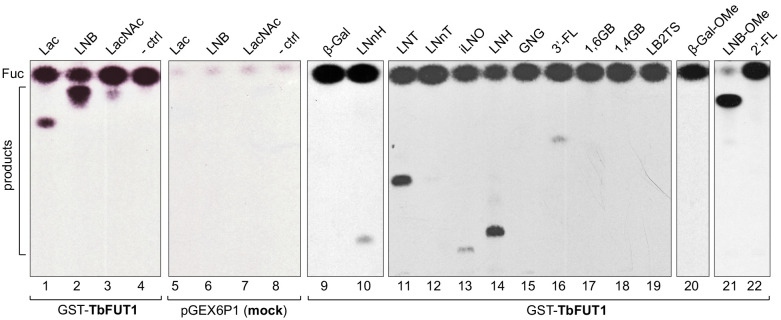
Figure 2. Recombinant GST-TbFUT1 transfers [3 H]Fuc to a variety of sugar acceptors.
Each assay used 2 μg of purified GST-TbFUT1, GDP-[3H]Fuc, and 1 mM of acceptor. Reaction products were desalted and separated by silica High Performance Thin Layer Chromatography (HPTLC) and detected by fluorography. LNB, LNB-OMe, and LNB-terminating structures were the best acceptors tested. The acceptor abbreviations above each lane are defined in Table 1. -ctrl: negative control reaction without acceptor (lane 4) or with buffer alone (lane 8).
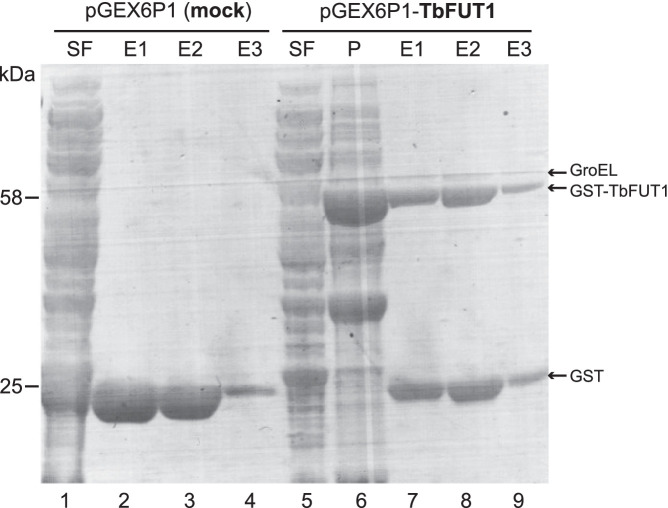
Figure 2—figure supplement 1. Purification of recombinant GST-TbFUT1.
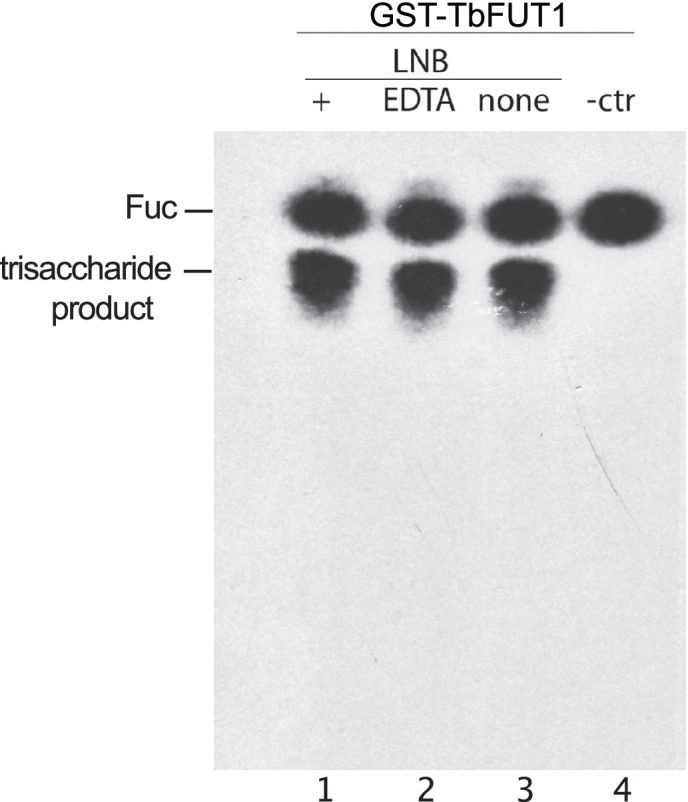
Figure 2—figure supplement 2. TbFUT1 activity is independent of divalent metal cations.