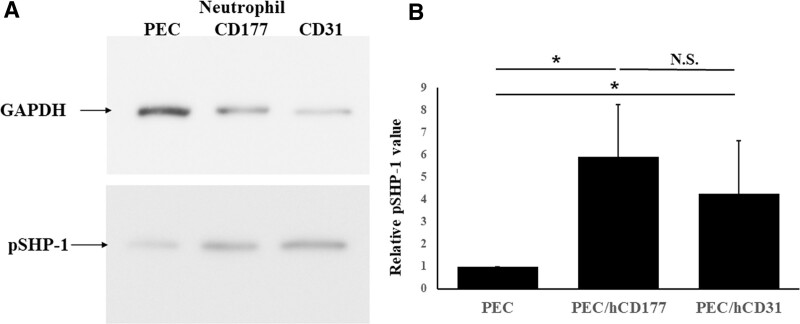
FIGURE 5.
SHP-1 phosphorylation induced by hCD177 and hCD31. PEC, PEC/hCD177, and PEC/hCD31 were seeded in gelatin-coated 6 well plates at a concentration of 1.6 × 105 cells/well and cocultured with neutrophils at a concentration of 1.6 × 106/well. Proteins were extracted from neutrophils after 30 min coculturing in the presence of 200 nmol/L PMA and loaded into 10% SDS-PAGE gel, followed by the transfer to PVDF membranes. The blot was stained with antiphosphorylated SHP-1 mAb and HRP-coupled secondary antibody. Representative data for 7 experiments are shown in (A). The extent of phosphorylation of SHP-1 by hCD177 and hCD31 was quantitatively evaluated using the Image J software program. All results of relative phosphorylated SHP-1 values are shown as the mean ± SEM (B). *P < 0.05. HRP, horseradish peroxidase; N.S., no significant difference, n = 7; PEC, porcine endothelial cells; PMA, phorbol 12-myristate 13-acetate; PVDF, polyvinylidene difluoride; SHP-1, src homology region 2 domain-containing phosphatase 1.