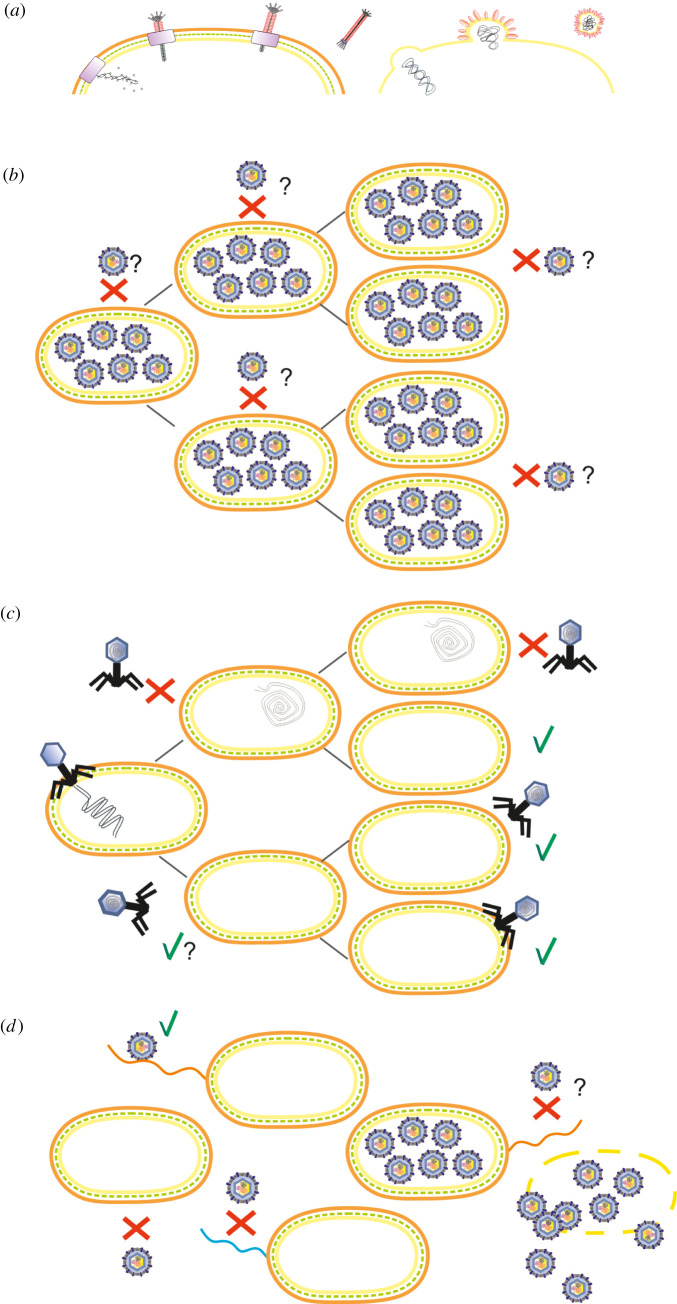
Figure 1.
Schematic presentation of alternative phage infection strategies. (a) Productive, chronic infection in which progeny phage particles are released by extrusion (left) or by budding (right) through the cell membrane without lysing the host bacterium. (b) Non-productive, chronic infection, in which large amounts of intracellular phage particles are produced without host lysis. The intracellular phage particles may confer superinfection exclusion. (c) Pseudolysogeny, displaying a stalled phage development stage in which the unintegrated phage genome, is asymmetrically passed on to daughter cells. Daughter cells may become resistant (indicated by red crosses) to secondary infections through the inheritance of the phage genome or, as in the case of phage P22, immunity factors [26]. Upon the dilution of the immunity factors through subsequent cell divisions, the resistant subpopulation ultimately becomes sensitive to phage infections (indicated by green ticks). (d) Population-level carrier state life cycle describing mixtures of phages and bacteria in a more or less stable equilibrium, due to the presence of sensitive variants (that are susceptible to phage infection and thus prone to phage-induced lysis) among resistant bacteria. Phage-resistant subpopulation may result from genetic and physiological changes of the host cells. In the figure, the lack of (no pilus) or phenotypic change (pilus mutant coloured in blue) of the phage receptor (wild-type pilus coloured in orange) or the presence of intracellular phage particles has induced the phage resistance.