Figure 6. Verteporfin is absorbed by human patient GBM tissue in a phase 0 clinical trial.
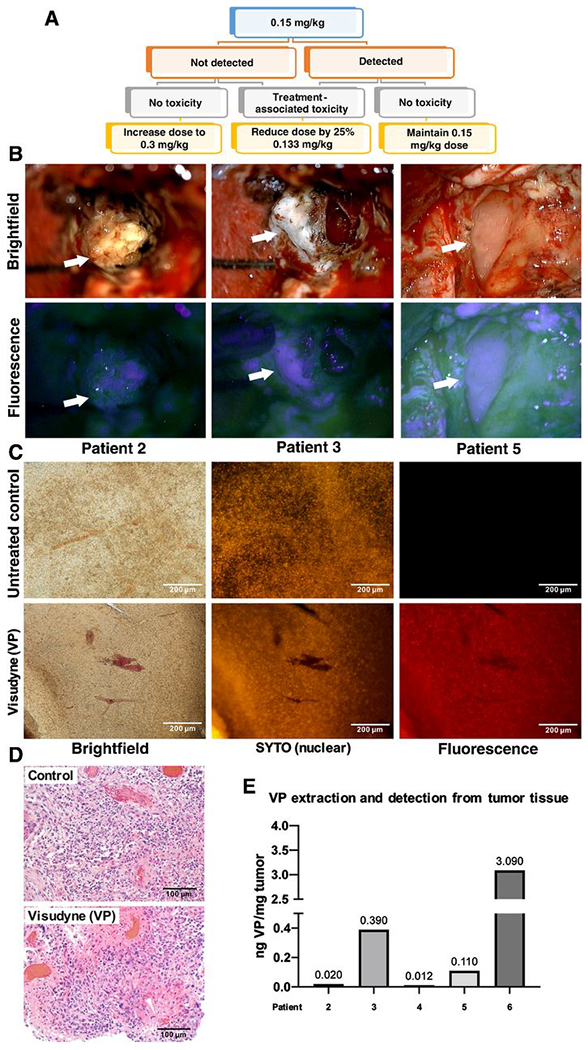
(A) Schematic of phase 0 clinical trial design. Participants received a single dose of Liposomal verteporfin (Visudyne) I.V. before surgery, at a starting of dose of 0.15 mg/kg at which verteporfin (VP) is administered to patients undergoing PDT for glaucoma. When verteporfin was not clearly detected in tumor cells at 0.15 mg/kg in the first participants, the following participants received 0.3 mg/kg.
(B) Intraoperative microscopy was accomplished with variable magnification over 2-40x to optimize visualization of tissues and structures of interest. Fluorescence was accomplished by adapting the surgical operating microscope with a filter set that modified the standard xenon light to provide fluorescence excitation in the wavelength range 390-440nm and for observation in the 600-700nm range. Arrows denote various portions of tumor under standard white light (upper) and with fluorescence (lower), respectively.
(C) Representative tumor tissue slice from a trial participant and a control patient who was not treated with verteporfin (left). Fluorescent nucleic acid counterstain (SYTO) was used to visualize cell nuclei (middle), and fluorescence using a verteporfin-specific excitation and emission filter is shown on the right.
(D) Micrographs of H&E staining on matched tissue from the tumor in (C) to visualize tumor cellularity in the region used to visualize verteporfin fluorescence.
(E) Representative tumor tissue from patients was digested, and solubilized verteporfin extracts were analyzed using mass spectrometry according to methods. Extracted verteporfin was detected at 0.012-3.090 ng/mg tumor in treated patients.
