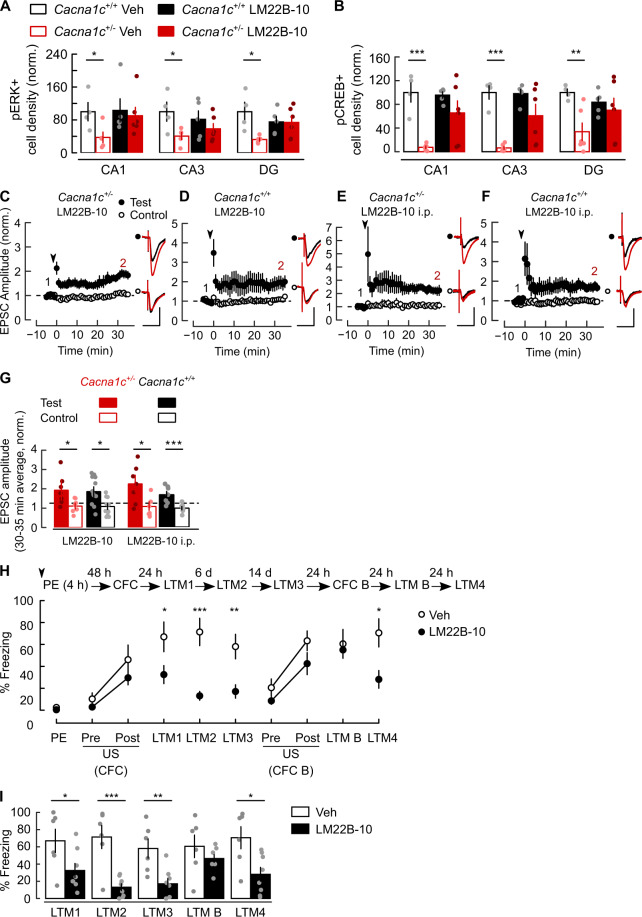
Fig. 5. The TrkB/TrkC receptor agonist LM22B-10 rescued dorsal hippocampal ERK-CREB signalling, synaptic plasticity, and LI of CFC in Cacna1c+/− rats.
Systemic administration of the TrkB/TrkC agonist LM22B-10 (25 mg/kg, i.p.) restored the baseline levels of pERK (A) and pCREB (B) in dorsal hippocampus of Cacna1c+/− rats; pERK genotype × treatment: F(1,16) = 4.567, p = 0.048; genotype effect, vehicle-treated (Veh): F(1,16) = 12.866, p = 0.002; LM22B-10-treated: F(1,16) = 0.615, p = 0.444; pCREB genotype × treatment: F(1,17) = 5.239, p = 0.035; genotype effect, Veh: F(1,16) = 21.319, p = 0.000, and LM22B-10: F(1,16) = 2.485, p = 0.133. There was no significant treatment effect in Cacna1c+/+ animals (pERK: F(1,21) = 0.54, p = 0.47; pCREB: F(1,21) = 0.98, p = 0.33). Bars represent summary of immunopositive cell densities in CA1, CA3 and dentate gyrus (DG) of dorsal hippocampus, normalized to average Cacna1c+/+ values. Examples of immunoreactive sections are shown in Fig. S14. Animals were sacrificed 60 min after i.p. administration of drug or vehicle and processed for both ERK and CREB; sample sizes for Cacna1c+/+: LM22B-10 n = 5, Veh n = 4; Cacna1c+/−: LM22B-10 n = 6, Veh n = 5 (pERK) and n = 6 (pCREB). C–G LM22B-10 rescued SC-CA1 LTP induction with TBP in slices from Cacna1c+/− rats, either during bath application (2 µM, C) or after i.p. bolus (25 mg/kg, E). LM22B-10 had no effect on TBP-induced SC-CA1 LTP in wild-type slices, either during bath application (D) or after i.p. bolus (F). Insets: 5 min average EPSC waveforms before (1, black) and 30–35 min after (2, red) LTP induction; scale bars: 50 pA, 50 ms. G Summary of change in normalized mean EPSC amplitude at 30–35 min after LTP in (C–F). Pairwise test vs control, C p = 0.034, n = 12(4); D p = 0.032, n = 7(4); E p = 0.044, n = 7(4); F p = 0.008, n = 7(5); sample sizes given as cells(animals). H Intrahippocampal infusion of LM22B-10 before context pre-exposure rescued LI of CFC in Cacna1c+/− animals. Schematic: behavioural paradigm in Fig. S1 extended with memory retrieval trials in LI context (LTM3, LTM4), alternative context training (CFC B) and retrieval (LTM B). Arrowhead: bilateral infusion of 1 µl LM22B-10 (2 µM, n = 8) or vehicle (Veh, n = 6) in dorsal hippocampus 60 min before PE. The plot shows the freezing response in all sessions. I Summary of freezing response at LTM trials. The freezing responses in LM22B-10 and Veh groups were different (treatment × stage: F(5,60) = 5.205, p = 0.002). Compared to the vehicle group, LM22B-10 animals showed reduced freezing responses only in the LI context (between-subject contrasts vs Veh, LTM1: F(1,11) = 4.162, p = 0.033, LTM2: F(1,11) = 18.467, p = 0.0005, LTM3: F(1,11) = 9.602, p = 0.005, LTM4: F(1,11) = 6.886, p = 0.012, LTM B: F(1,11) = 0.138, p = 0.358). All data are presented as means ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, two-way repeated measures ANOVA then pairwise post-hoc comparison with Bonferroni correction (A, B, G, H) and two-way ordinal regression (ANODE) then Tukey-adjusted post-hoc comparisons (colour figure online).