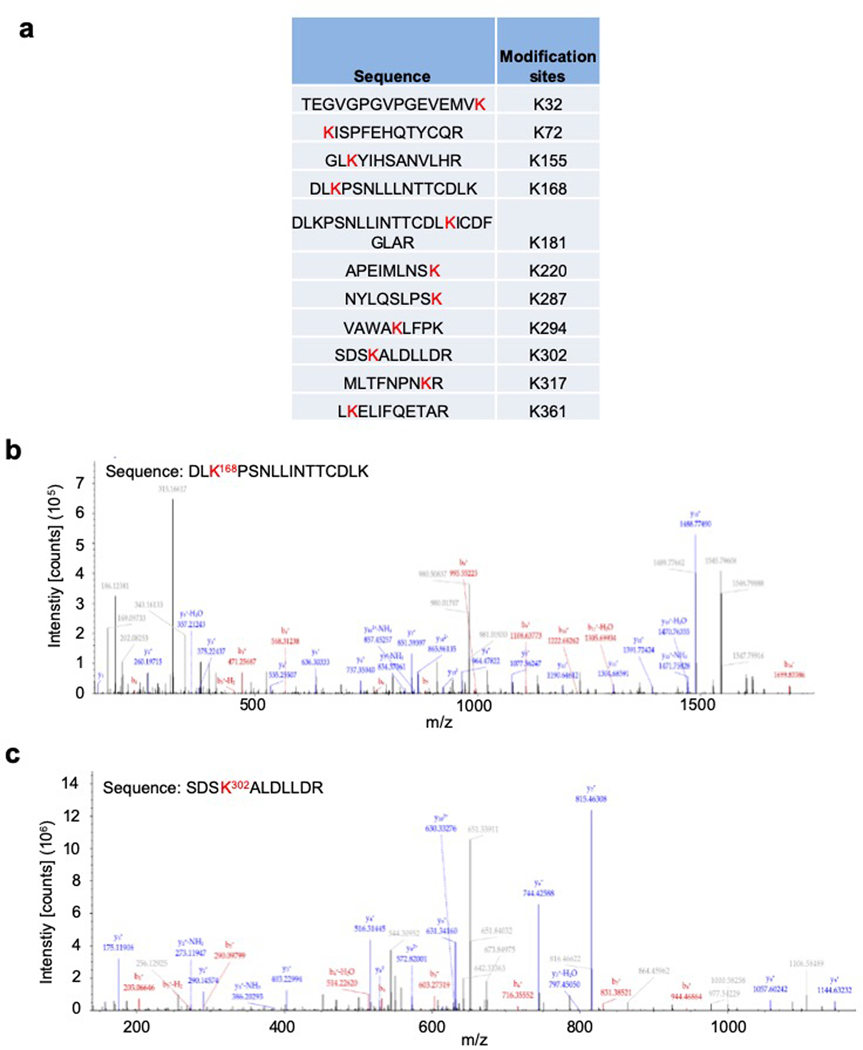
Extended Data Fig. 3. Mass-spectrometry analysis of ERK1 for potential ubiquitination sites.
Flag-ERK1 protein co-expressed with HA-TRIM15 in HEK293T cells (Flag-ERK1Ub) were purified and analyzed by mass spectrometry. Shown are ubiquitination sites of ERK1 (a), and mass spectrum of peptides surrounding K168 (b) and K302 (c). Ubiquitination at K302 was detected when a relatively small amount of Flag-ERK1Ub protein was used, while ubiquitination at K168 was detected only when a relatively large amount of Flag ERK1Ub protein was used, suggesting that ubiquitination at K302 was more abundant than that at K168.