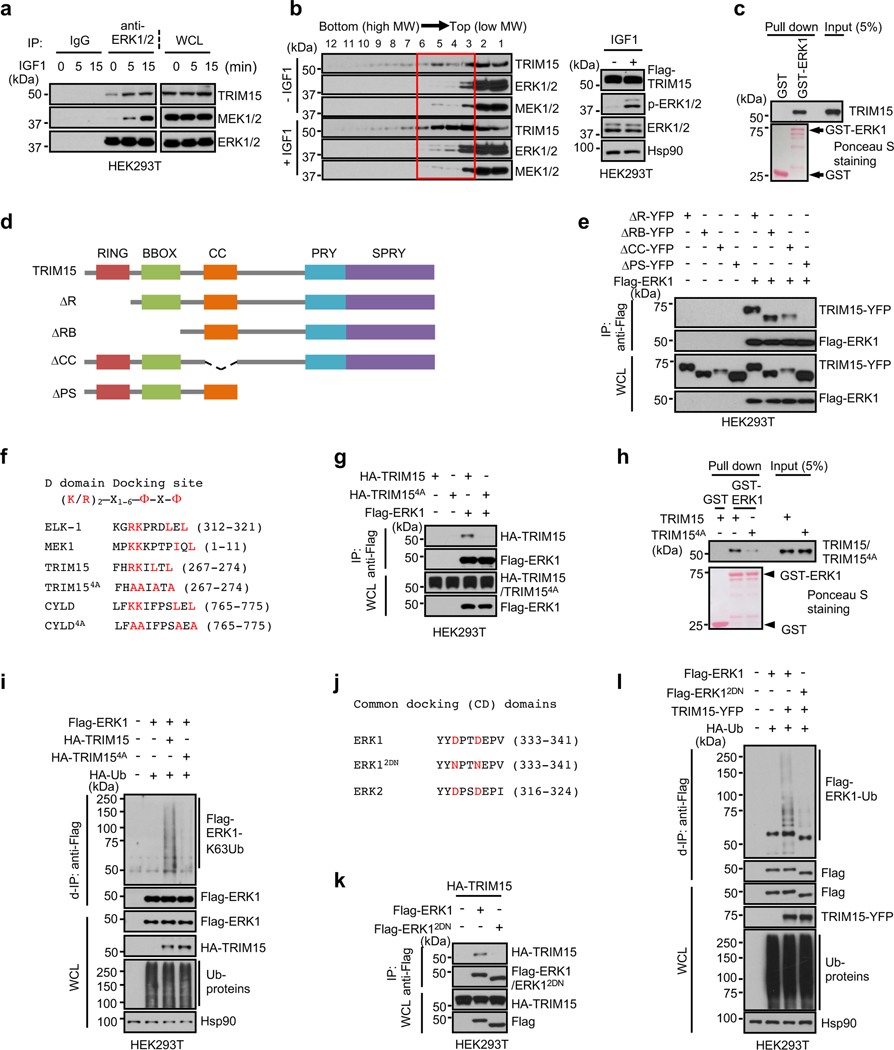
Fig. 3 |. TRIM15 interacts with ERK1/2 via conserved domains.
a, Interaction of ERK1/2 with TRIM15 and MEK1/2 in cells. Serum-starved HEK293T cells were treated with IGF1 for the indicated times. Cell lysates were immunoprecipitated with control IgG or anti-ERK1/2 antibody. Immunoprecipitates and WCL were analyzed by Western blot.
b, HEK293T cells transfected with Flag-TRIM15 were serum-starved and treated with or without IGF1 for 15 min. Cell lysates were centrifugated in sucrose gradient. Fractions (left) and unfractionated cell lysates (right) were analyzed by Western blot.
c, h, TRIM15-ERK1 interaction in vitro and its dependence on TRIM15 D-domain docking site. Purified Flag-TRIM15 (c,h) or Flag-TRIM154A (h) was incubated with GST or GST-ERK1 conjugated on beads. Pulldown samples and 5% of input were analyzed by Western blot and Ponceau S staining.
d, Schematic illustration of the full length TRIM15 and its deletion mutations.
e, The TRIM15 PRY-SPRY region is required for interaction with ERK1. Cell lysates derived from HEK293T cells transfected with the indicated Flag-ERK1 protein were analyzed by co-IP assay with anti-Flag mAb (M2) beads, followed by immunoblot.
f, The D domain-docking sites in ELK-1 and MEK1 and putative D domain-docking sites in TRIM15 and CYLD, with conserved residues indicated in red color. In TRIM154A and CYLD4A, four conserved residues were replaced with Ala. Amino acid numbers are indicated.
g, k, HEK293T cells were transfected with the indicated Flag-ERK1 and HA-TRIM15 plasmids. Anti-Flag immunoprecipitates and WCL were analyzed by Western blot.
i, l, TRIM15-mediated ERK1 ubiquitination is dependent on their stable interaction via conserved domains. HEK293T cells transfected with Flag-ERK1 together with HA-TRIM15 or HA-TRIM154A (i), or with TRIM15-YFP together with Flag-ERK1 or Flag-ERK12DN (l), were analyzed for ERK1 ubiquitination, total ubiquitinated proteins, and protein expression by d-IP and Western blot.
j, Common docking (CD) domains in ERK1 and ERK2. In ERK12DN, the two conserved Asp residues were replaced with Asn.
Assays in panels a-c, e, g-i, k and l have been performed two times with similar results.