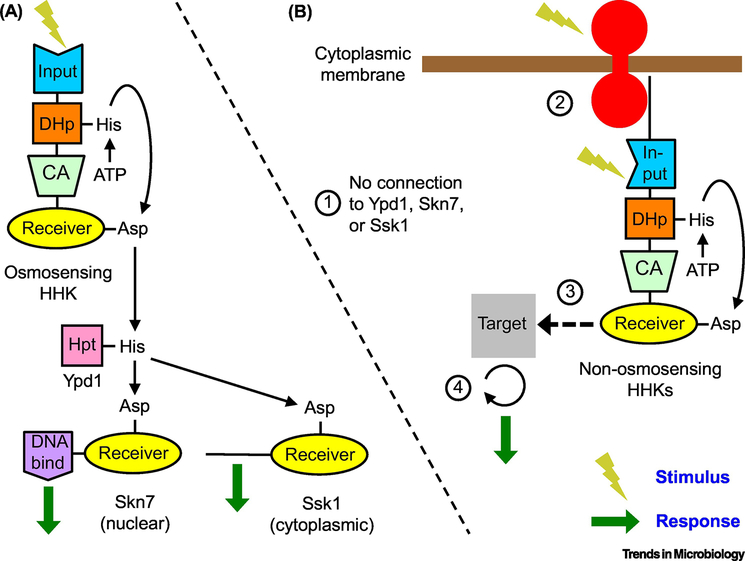
Figure 3. Reimagined fungal TCSs.
A) Canonical: Osmosensing TCS functions as a multi-step phosphorelay from the osmosensing HHK to Ypd1 to Skn7 and Ssk1 essentially as in S. cerevisiae. B) Hypothetical: Non-osmosensing TCSs generally do not function as phosphorelays, which leads to four predictions. (1) Because there is no phosphorelay, the phenotypes resulting from removal of non-osmosensing HHKs will be different than the phenotypes resulting from removal of Ypd1, Skn7, or Ssk1. (2) The HHKs can detect cytoplasmic stimuli via their own input domains, or bind to transmembrane receptors to process external stimuli. Fungal HHKs have substantial N-terminal regions with no identifiable domains, which could serve to bind transmembrane receptors. Furthermore, binding of one type of HHK to a family of receptors could integrate multiple inputs in a manner analogous to bacterial chemoreceptors. (3) The receiver domain of the HHKs binds to downstream targets in a phosphorylation dependent manner. Alternatively, HHKs might interact with downstream targets via non-TCS phosphorylation (e.g., transfer from the HHK receiver domain to a His residue on a non-TCS protein, or phosphorylation mediated by the Ser/Thr kinase domains of Group X and XVI HHKs). (4) Because phosphotransfer within HHKs does not result in signal amplification, some targets of HHKs may have amplification capability (e.g. kinase cascades, generation of second messengers, etc.). Alternatively, activation of multiple HHKs by a single transmembrane receptor as predicted in (2) could result in signal amplification. Reversible and dephosphorylation reactions are not shown for clarity.