Figure 4. Hypothalamic circuits for feeding are distinguished by calorie-specific versus indiscriminate food intake.
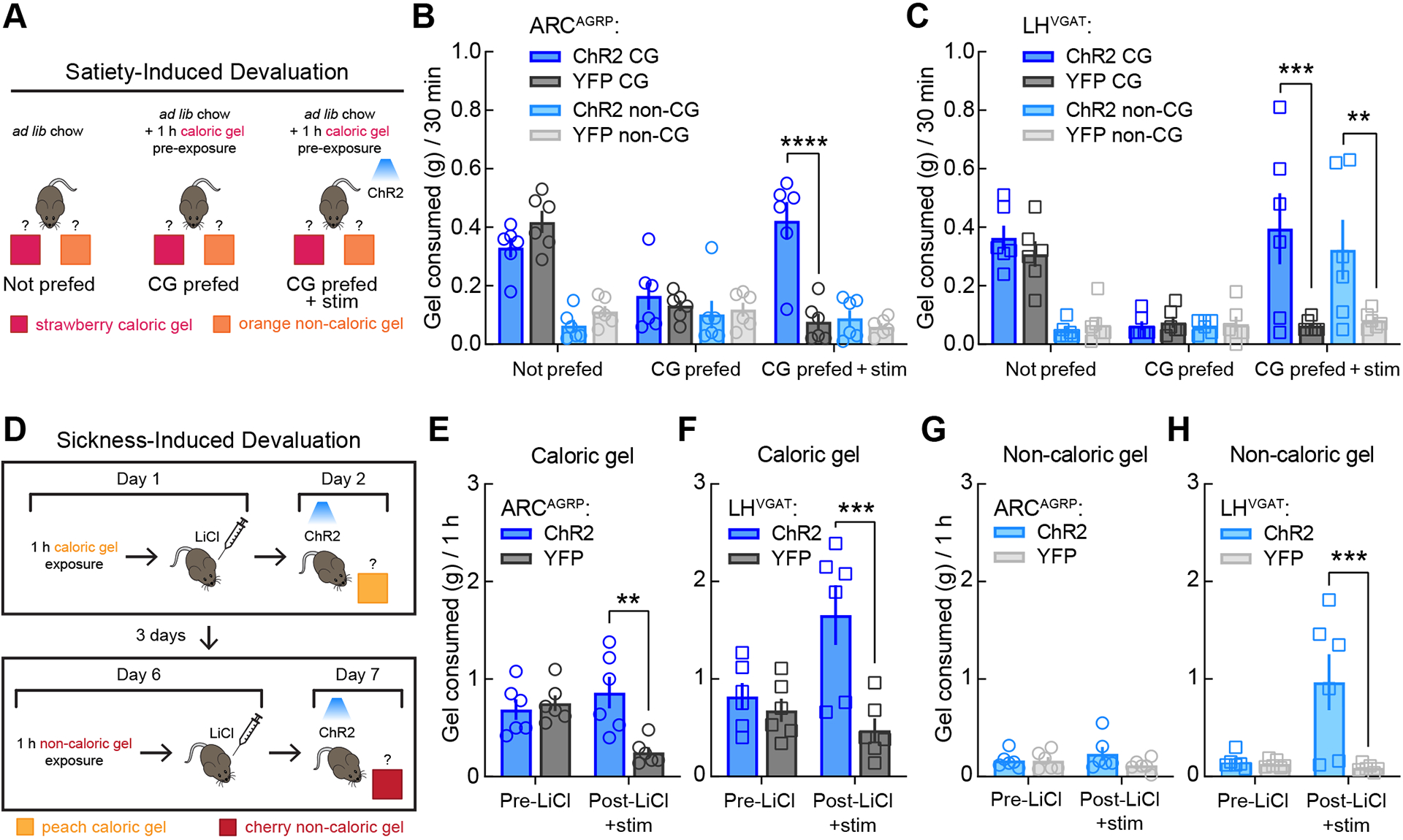
(A) Schematic representation of satiety-induced devaluation. In test 1 (Not prefed), mice were housed with ad libitum chow and had 30-min simultaneous access to the caloric and non-caloric gels. In test 2 (CG prefed), mice had 1 h of access to caloric gel in the home cage prior to the 30-min choice session. Test 3 (CG prefed + stim) was the same as test 2 except that photostimulation was delivered during the 30-min choice session. n = 6 mice per group for all tests.
(B) ARCAGRP activation triggered calorie-specific gel intake following satiety-induced devaluation in the two-gel choice assay. A three-way mixed-model ANOVA revealed a significant test × calorie × group interaction, F(2, 20) = 7.92, p = 0.0029) and that among the two-factor interactions, the test × group interaction (p = 0.0004) accounted for the most variation. Thus, follow-up two-way (test × group) mixed-model ANOVAs within each gel revealed a significant test × group interaction for caloric gel (F(2, 20) = 13.47, p = 0.0002) but not non-caloric gel (F(2, 20) = 1.13, p = 0.34). Bonferroni’s post-tests revealed that all mice displayed a significant decrease in caloric gel intake following 1 h caloric gel pre-exposure (CG prefed) as compared to normal ad libitum fed conditions (Not prefed, p = 0.0001) and that photostimulation increased caloric gel intake following devaluation in ARCAGRP:ChR2 as compared to ARCAGRP:YFP control mice (CG prefed + stim, ****p < 0.0001).
(C) LHVGAT activation triggered indiscriminate gel intake following satiety-induced devaluation in the two-gel choice assay. A three-way mixed-model ANOVA revealed no significant test × calorie × group interaction (p = 0.63) but that among the two-factor interactions, the test × group interaction (p = 0.004) accounted for the most variation. Thus, follow-up two-way (test × group) mixed-model ANOVAs within each gel revealed significant test × group interactions for both caloric gel (F(2, 20) = 4.95, p = 0.018) and non-caloric gel (F(2, 20) = 6.39, p = 0.0072). Bonferroni’s post-tests revealed that all mice displayed a significant decrease in caloric (p = 0.0005) but not non-caloric (p > 0.99) gel intake following 1 h caloric gel pre-exposure, but that photostimulation increased both caloric (***p = 0.0008) and non-caloric (**p = 0.0019) gel intake following devaluation in LHVGAT:ChR2 as compared to LHVGAT:YFP control mice.
(D) Schematic representation of sickness-induced devaluation. Mice were given access to caloric gel for 1 h followed by i.p. injection with LiCl. The following day, mice were exposed to the caloric gel during optogenetic stimulation and caloric gel consumption was measured. Three days later, LiCl-induced devaluation was repeated with a non-caloric gel. n = 6 mice per group for all tests.
(E) ARCAGRP activation triggered calorie-specific gel intake following LiCl-induced devaluation in single-gel test sessions (F(1, 10) = 7.47, p = 0.021; Bonferroni’s post-test **p = 0.0014).
(F) LHVGAT activation evoked indiscriminate gel intake following LiCl-induced devaluation in single-gel test sessions (caloric gel: F(1, 10) = 11.78, p = 0.0064; Bonferroni’s post-test: ***p = 0.0005).
(G) ARCAGRP activation did not affect non-caloric gel intake (F(1, 10) = 2.18, p = 0.17).
(H) LHVGAT activation evoked indiscriminate non-caloric gel intake following LiCl-induced devaluation in single-gel test sessions: F(1, 10) = 9.13, p = 0.013; Bonferroni’s post-test: ***p = 0.0007).
